Antidiabetic Activity of Momordica charantia Extracts Through Incretin Pathway in Streptozotocin-Nicotinamide Induced Diabetic Rat Depends on Dose Differences
-
 Fadhol Romdhoni , Muhammad
Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia; Department of Pharmacology, Faculty of Medicine, Universitas Muhammadiyah Purwokerto, Banyumas, Indonesia, Tel: +62 81334722484; E-mail: romdhoni@student.uns.ac.id
Fadhol Romdhoni , Muhammad
Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia; Department of Pharmacology, Faculty of Medicine, Universitas Muhammadiyah Purwokerto, Banyumas, Indonesia, Tel: +62 81334722484; E-mail: romdhoni@student.uns.ac.id
-
Doewes , Muchsin
-
Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia
-
Department of Pharmacology, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia
-
Soetrisno , Soetrisno
-
Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia
-
Department of Obstetrics and Gynaecology, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia
-
Puspita Febrinasari , Ratih
-
Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia
-
Department of Pharmacology, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia
Abstract: Background: This study addresses the increasing prevalence of type 2 Diabetes Mellitus (T2DM) and the need for alternative, cost-effective medications. The research problem focuses on the need to further study the dose effectiveness of Momordica charantia Extracts (MCE) on T2DM parameters, including Glucagon-Like Peptide 1 (GLP-1), Dipeptidyl Peptidase 4 (DPP-4), alpha glucosidase, glucosetransporter 5 (GLUT5), and pancreatic tissue histopathology.
Methods: The methodology employed an experimental research design with 30 Wistar rats divided into six groups, each receiving different inductions and doses. Parameters were measured using Elisa, and histological analysis of pancreatic tissue was conducted using HE staining.
Results: The Kruskal-Wallis test revealed significant differences in each group for the GLP-1 (p=0.003). However, the DPP4 test suggested a lack of significant difference in each group (p=0.192), and the GLUT5 test showed insignificant changes between each group (p=0.119). The ANOVA analysis on alpha-glucosidase revealed no statistically significant differences among the groups (p=0.202). Additionally, a qualitative examination of the histological analysis of pancreatic tissue indicated an improvement in the condition of the pancreatic tissue.
Conclusion: MCE can increase GLP-1 levels, lower DPP-4, lower alpha-glucosidase, and raise GLUT5. However, there are no significant differences between other groups and the morphology of pancreatic tissue in rat model T2DM at a dose of 300 mg/kg.
Introduction :
Type 2 Diabetes Mellitus (T2DM) is characterized by chronic hyperglycemia as a result of impaired insulin action and secretion 1. Insulin resistance results in a decrease in glucose uptake in skeletal muscle and adipose tissue which then results in an increase in demand for insulin secretion from pancreatic beta cells, but the pancreatic beta cells fail to meet the increased demand for insulin secretion 2. In 2015, the incidence of T2DM was around 415 million people in the age range between 20 and 79 years and is predicted to increase in 2030 by 578 million people and in 2045 by 700 million people, and is ranked sixth as the leading cause of death in the world 3. Indonesia is in third place with the most T2DM cases among people aged 20-79 years with the number of cases in 2019 reaching 10.7 million people, which is predicted to increase in 2030 to 13.7 million people and in 2045 to 16.6 million people 4.
The diagnostic criteria for T2DM are based on venous blood samples examined in the laboratory. A fasting blood glucose (GDP) level of 7.0 mmol/L correlates most closely with a 2 hr Post Prandial (2JPP) blood glucose value ≥11.1 mmol/L in a 75 g Oral Glucose Tolerance Test (OGTT), and each can predict the development of retinopathy complications 5. The Hemoglobin A1c (HbA1c) examination is claimed to be very specific, but less sensitive for diagnosing diabetes than traditional glucose criteria. Some of the advantages of HbA1c testing are that it can be measured at any time of the day and is more convenient than GDP or 2 JPP in 75 g OGTT. HbA1c testing also avoids the problem of daily variability in glucose values because it reflects the average blood glucose over the previous 2 to 3 months 6.
The pathogenesis of T2DM is multifactorial. Understanding the complex pathophysiology of T2DM can provide the right approach in providing treatment. The pathophysiological development of DMT2 from the triumvirate (pancreatic beta cell failure, insulin resistance in muscle and liver) to the Ominous octet was first described by DeFronzo in 2009. The approach taken from the Ominous octet was through the digestive tract and pancreatic beta cells. The digestive tract is associated with incretin hormones and fructose transport via Glucosetransporter 5 (GLUT5), while pancreatic beta cells play an important role in insulin secretion 7. Incretin is a hormone produced by intestinal cells whose secretion takes place immediately after a person consumes food. The type of incretin that has the most potent effect is Glucagon-Like Peptide-1 (GLP-1) 8. GLP-1, an endocrine hormone of 30 amino acids produced in enteroendocrine L cells, is an important physiological regulator of metabolic control and is an important hormone that stimulates insulin secretion from pancreatic cells. The active GLP-1 hormone is then degraded by the enzyme Dipeptidyl Peptidase-4 (DPP-4) in the kidney brush border membrane, hepatocyte cells and capillary endothelial cells so that DPP-4 inhibitor drugs are needed 9. The GLP-1 hormone and the GLP-1R receptor are therapeutic targets for treating T2DM, so drugs that modulate the hormone and its receptor are used, such as Exenatide, Liraglutide, Vildagliptin, and Sitagliptin. Apart from having a therapeutic effect, these drugs have side effects including fluid retention, weight gain and headaches 10. The side effects and high prices of these drugs require looking for alternative drugs for treating T2DM. Since ancestral times, medicinal plants and their extracts have been used as the first line to treat diabetes, one of which is bitter melon (Momordica charantia) 9.
Momordica charantia (M. charantia) (commonly called bitter melon, cerassee, goya, bitter apple, bitter gourd, bitter squash, balsam-pear, karavila and many more names) is a tropical and subtropical vine of the family Cucurbitaceae, widely grown in Asia, Africa, and the Caribbean for its edible fruit. Its many varieties differ substantially in the shape and bitterness of the fruit. Bitter melon originated in Africa, where it was a dry-season staple food of Kung hunter-gatherers. Wild or semi-domesticated variants spread across Asia in prehistory, and it was likely fully domesticated in Southeast Asia. It is widely used in the cuisines of East Asia, South Asia, and Southeast Asia 11.
M. charantia, which is in the Cucurbitaceae family and Genus momordica, is a tropical plant used to treat diabetes mellitus. The mechanisms of action of bitter melon include protecting pancreatic beta cells, increasing insulin, inhibiting intestinal α-glucosidase, inhibiting glucose transport, activating AMPK, and reducing gluconeogenesis 12. The charantin content extracted from bitter melon showed hypoglycemic effects in normal and diabetic rabbits. At a dose of 375 mg/kg, methanol extract of bitter melon reduced fasting blood glucose after 12 hr in alloxan-induced diabetic rats. In other studies, the results of reducing blood glucose, cholesterol and triglycerides through reducing PKC-β activity were also reported in streptozotocin-induced diabetic rats when bitter melon juice was given at 6 ml/kg 13. N-hexane fractionation and ethanol extract from bitter melon fruit have the effect of reducing malondialdehyde and simultaneously repairing streptozotocin-induced liver damage in rats 14,15. Protein extract from bitter melon inhibits alpha-amylase and alpha-glucosidase in vitro and lowers blood glucose levels in vivo equivalent to Acarbose when administered orally to Streptozotocin (STZ)-induced diabetic rats 16.
Materials and Methods :
This research is experimental research with the Post Test Only Control Group Design approach. Examination of markers GLP-1, DPP 4, alpha glucosidase, and GLUT5 using Elisa kit reagents from BT Lab®. Momordica charantia collected from Medicinal Plant and Traditional Medicinal Medicine Research and Development Center, Karanganyar, Central Java, Indonesia (herbarium code: TAMA) and was processed and determined by PT Lansida, Yogyakarta, Indonesia. This study used 30 male Wistar rats aged 3-4 months with a body weight of 200-250 g.
Preparation of plant material: The bitter melon fruit was acquired and analyzed by PT Lansida, located in Yogyakarta. The fruit was thereafter crushed and soaked in a solution consisting of 96% ethanol for a period of 3×24 hr. The maserate was subjected to evaporation using a rotary evaporator, maintaining a temperature below 40°C, until a viscous and concentrated extract was obtained that retained its fluidity.
Induction of diabetic state in wistar rat: Following the acclimatization procedure, the mice were assessed for weight and it was verified that they were exhibiting rapid locomotion and showed no indications of disease. The mice were administered an intraperitoneal injection of at a dosage of 230 mg/kg Body Weight (BW). After 20 min, another intraperitoneal injection of STZ was administered at a dosage of 65 mg/kg BW. Following a period of 5 days, the produced extract was administered.
Treatment of animals: Group 1 (normal control) received standard feed. Group 2 (positive control) was given STZ-Nicotinamide (NA) and standard feed. Group 3 (negative control) was given STZ-NA, vildagliptin, and standard feed. Group 4 (treatment group 1) was induced with STZ-NA and 75 mg/kg BW Momordica charantia Extracts (MCE)+standard feed. Group 5 (treatment group 2) was induced with STZ-NA and 150 mg/kg BW MCE+standard feed. Group 6 (treatment group 3) was induced with STZ-NA and 300 mg/kg BW MCE+standard feed.
Blood sampling: Blood collection was carried out via retroorbital which is described as follows:
a) Using a haematocrit capillary tube was inserted in such a way that it reached the retroorbital plexus region.
b) The blood that came out was collected in an Eppendorf tube.
c) The blood in the tube was put into a centrifuge and centrifuged for 10 min at a speed of 12,000 rpm to separate the serum from other parts of the blood. Next, the separated blood sera was used for measurements.
GLP-1-1, dipeptidyl peptidase-4, alpha glucosidase, and glucose transporter 5 levels were quantified using the Enzyme-Linked Immunosorbent Assay (ELISA) technique. The ELISA kit utilized is the BT lab brand, accompanied with specific information: E0918Ra for GLP1, and E0226Ra for DPP4. Alpha glucosidase was measured using Mybiosource brand: Catalogue number MBS722447 for alpha glucosidase and Catalogue number MBS9345383 for glucose transporter5.
Histopathology of pancreas: Performing histological analysis of the pancreas via Haematoxylin and Eosin (H&E) staining.
Results :
The research yielded significant findings that offer a full understanding of the sample's features, the correlations between key factors, and the theoretical implications. The findings of this study not only have a significant impact on the field of medicine, but also serve as a solid foundation for making informed decisions at the practical and clinical level.
The study utilized a sample of 30 male Wistar white mice, with an age range of 3-4 months and a weight range of 200-250 g. The number was arbitrarily divided into 6 groups, each containing 5 animals. Following the acclimatization procedure, it was verified that the mice were exhibiting rapid locomotion and showed no indications of sickness. The mice received an intraperitoneal injection of NA at a dosage of 230 mg/kg BW. After 20 min, they were then intraperitoneally stimulated with STZ at a dosage of 65 mg/kg BW. After a duration of 5 days, the produced extract was administered 17-19. In order to guarantee the efficacy of STZ-NA induction, blood glucose levels were assessed prior to and following induction, as documented in table 1.
Based on the results of a normality test using the Shapiro-Wilk test from table 1, it was determined that the data followed a normal distribution. Additionally, the homogeneity test indicated that the data was homogeneous. Consequently, an ANOVA test was conducted to compare the pre- and post-previous blood glucose levels after induction. The obtained results showed a p-value greater than 0.05, indicating a significant difference between the two groups. The efficacy of induction is seen in figure 1, which displays a rise in blood glucose levels, hence confirming the successful establishment of an animal model for T2DM. Figure 1 clearly demonstrates a substantial rise in blood glucose levels across all treatment groups.
GLP-1: GLP-1 is an incretin hormone that is synthesized by L cells in the digestive system through the transcription of the proglucagon gene. Similar to Glucagon, GLP-1 experiences restricted breakdown throughout the process of synthesis. The GLP-1 levels in table 2 was assessed for normality using the Shapiro-Wilk test. The results indicated that the data in each group followed a normal distribution (p>0.05). However, when testing for homogeneity, the data did not meet the criteria (p<0.05). Therefore, a non-parametric test was conducted. The Kruskal-Wallis test is a non-parametric statistical test. The Kruskal-Wallis test findings indicate statistically significant differences among each group, with a p-value of less than 0.05, as presented in table 3.
DPP4: Dipeptidyl Peptidase-4 (DPP-4) is a ubiquitous enzyme that acts on incretin hormones, particularly GLP-1 and Gastric Inhibitory Peptide (GIP), which maintain glucose homeostasis by increasing insulin secretion and decreasing glucagon secretion. The normality of the DPP4 levels in table 4 was tested using the Shapiro-Wilk test, which revealed that the data in each group were normally distributed (p>0.05). However, the homogeneity test showed that the data were not homogenous (p<0.05); therefore, requiring the use of the non-parametric Kruskal-Wallis test. The Kruskal-Wallis test results indicate that there is no significant difference among the respective groups, with p>0.05, as shown in table 5.
Alpha glucosidase: Alpha glucosidase is an enzyme involved in the hydrolysis of carbohydrates into glucose within the gastrointestinal system. This enzyme has the ability to elevate glucose levels in the bloodstream. The normality of the alpha glucosidase in table 6 was assessed using the Shapiro-Wilk test. The results indicated that the data in each group followed a normal distribution (p>0.05). Furthermore, the homogeneity test confirmed that the data was homogeneous (p>0.05). Subsequently, the ANOVA test was conducted as the conditions for normality and homogeneity were satisfied. The ANOVA test results indicate that there are no significant differences seen in either group, with a p-value greater than 0.05, as presented in table 7.
GLUT5: Glucose transporter 5 (GLUT5) is a protein that transports fructose from the intestinal lumen to enterocytes in the small intestine. It is located at the apical edge of enterocytes and facilitates the movement of fructose through facilitated diffusion. This process is possible because of the high concentration of fructose in the intestinal lumen. The normality of GLUT5 levels in table 8 was assessed using the Shapiro-Wilk test. The results indicated that the data in each group followed a normal distribution (p>0.05), except for group 1 which showed abnormal results (p<0.05). Subsequently, a test for data homogeneity was conducted, which confirmed that the data was homogeneous (p>0.05). Therefore, a non-parametric Kruskal-Wallis test was performed. The Kruskal-Wallis test findings indicate that there are no significant differences seen among the groups, with a p-value greater than 0.05, as presented in table 9.
Histopathology of pancreatic tissue: The research findings (Figure 2) indicate that the Islets of Langerhans in control mice exhibit few cavities or intercellular spaces, and include a significant number of pancreatic beta cells responsible for insulin production. Conversely, diabetic mice exhibit numerous cavities or intercellular spaces inside the Islets of Langerhans, resulting in a decrease in the quantity of insulin-producing pancreatic beta cells. Research data indicates that the most significant enhancement in pancreatic tissue occurs while providing bitter melon ethanol therapy at a dosage of 150 mg/kg BW.
The statistical analysis revealed a significant difference in the average degree of intrudis between the therapeutic dose of 150 mg/kg BW and the therapeutic doses of 75 and 300 mg/kg BW. The administration of a therapeutic dosage of 150 mg/kg BW to rats with diabetes mellitus resulted in the most significant enhancement in pancreatic tissue.
Discussion :
Bitter melon has several antidiabetic properties, such as safeguarding Islet of Langerhans cells, enhancing insulin production, suppressing alpha glucosidase in the gut, promoting hepatic glucose clearance, and diminishing gluconeogenesis. In addition, the bitter melon plant has the ability to elevate GLP-1 levels in the intestine, so effectively suppressing the activity of the DPP-4 enzyme. The sub-chronic injection of bitter melon leads to an upregulation of enteroendocrine L cell receptors, resulting in an elevation of GLP-1 production 20. Charantin is a chemical that can inhibit the DPP-4 enzyme 21. Charantin may also aid in reducing oxidative damage by counteracting free radical activity and promptly regulating β-cell apoptosis 13. Caravilagenin and triterpenoids found in bitter melon can enhance the insulin signalling pathway 22.
GLP-1: The study's findings revealed notable variations in the measurements of GLP-1 levels. The most elevated concentrations were seen in group 5 (administered with a 150 mg/kg BW ethanol extract of bitter melon fruit), surpassing the concentrations in group 3 (treated with conventional DPP4 inhibitor medications). These findings demonstrate that the ethanol extract derived from bitter melon fruit has the ability to elevate GLP-1 levels in rats with diabetes mellitus.
According to a study conducted by Chang et al in 2021, bitter melon fruit extract has been found to function as a secretagogue for GLP-1 in enteroendocrine cells. This effect is likely achieved by the activation of bitter taste receptors. Thus, bitter melon extract exerts effects on enhancing insulin sensitivity, replacing insulin, and stimulating the action of GLP-1 secretagogues in intestinal cells. Bitter melon extract may have a significant anti-diabetic effect on intestinal cells 23.
Additional studies on bitter melon fruit extract demonstrated a significant rise in tissue glycogen, serum insulin, and GLP-1 levels in diabetic Wistar mice (p<0.01). However, these effects were not significant (p>0.05) in normal mice. The fasting blood glucose and glycosylated hemoglobin levels were not statistically significant (p>0.05) in normal rats, but were statistically significant (p<0.01) in diabetic Wistar rats. The elevation of GLP-1 levels in both normal and diabetic mice administered bitter melon fruit extract can be attributed to the regeneration and proliferation of L cells through the binding to L cell receptors and inducing conformational alterations, consequently initiating a cascade of signal transduction events. M. charantia polar compounds induce depolarization in L cells via elevating intracellular Ca2+ levels, leading to the release of GLP-1. GLP-1 subsequently enhances the growth of beta cells and the release of insulin 20.
DPP4: The findings of this study indicate that there were no statistically significant variations observed between the groups when assessing Dipeptidyl Peptidase-4 levels. However, it is worth noting that the values in group 6, which received the highest dosage treatment, were comparatively lower than those in group 3, which utilized typical DPP4 inhibitor medications. These findings align with the research undertaken by Perumal et al in 2022, which discovered that a mixture of crude extracts from Taraxawm officinale (T. officinale) and M. charantia exhibited inhibitory properties against dipeptidyl peptidase-4 (DPP-4). The crude extract's DPP-4 inhibitory activity was evaluated utilizing a non-Cayman cell-based assay. This assay evaluates the activity of the DPP-4 enzyme by utilizing a fluorogenic substrate called Gly-Pro-Aminomethylcoumarin. The DPP-4 enzyme hydrolyzes peptide bonds in the substrate, resulting in the liberation of fluorescent Gly-Pro-Aminomethylcoumary groups. These groups are subsequently detected and studied using suitable excitation and emission wavelengths. The results indicate that Sitagliptin, the standard medication, exhibited the greatest inhibitory potency of 79.95±0.35% (p<0.05) compared to all crude extracts of T. officinale and M. charantia. Nevertheless, the crude extract of T. officinale exhibited the most potent inhibitory effect, particularly when using acetone solvent (44.85±0.44%), ethanol solvent (43.69±0.56%), and water solvent (28.15± 0.31%) 24.
A study employing computational techniques investigated the optimal antidiabetic peptide derived from the hypoglycemic P-polypeptide of M. charantia. The study evaluated the peptide's binding affinity and interaction patterns with four receptor proteins, specifically as an agonist for the insulin receptor and as an inhibitor for sodium-glucose cotransporter 1, dipeptidyl peptidase-IV, and glucose transporter-2. This evaluation was conducted using a molecular docking methodology. This receptor was bound by a total of thirty-seven peptides. Out of these, the eight most optimal ligands (specifically LIVA, TSEP, EKAI, LKHA, EALF, VAEK, DFGAS, and EPGGGG) were selected due to their strict adherence to Lipinski's rule of five and their demonstrated high quality 25.
Alpha glucosidase: The study's findings indicate that there were no significant variations in Alpha Glucosidase levels across the groups, as evidenced by a p-value over 0.05. The group administered with the normal treatment exhibited the lowest levels of alpha glucosidase, but the group treated with bitter melon fruit extract demonstrated higher levels compared to the standard drug. According to a study conducted by Bhat GA et al in 2022, it was found that bitter melon fruit extracted using any solvent can reduce the levels of alpha glucosidase. However, among the several solvents tested, ethanol had the least suppressive impact. The ethanol extract of bitter melon fruit has demonstrated its ability to lower blood glucose levels in male Kunming rats by blocking lactase activity, hence reducing the breakdown of sucrose, maltose, and lactose. The results suggest that saponin, a compound found in the ethanol extract of bitter melon fruit, has the ability to suppress the activity of alpha glucosidase in the small intestine of diabetic rat 26.
GLUT5: The findings of this study indicate that there were no statistically significant variations observed in GLUT5 levels across the different groups. However, it is worth noting that the values in the dosage therapy groups (groups 4, 5, and 6) were found to be greater compared to group 3, which received normal DPP4 inhibitor medicines. This contrasts with the findings of Ashley Dahlquist et al, who reported that flavonoids derived from the ethanol extract of bitter melon hinder the absorption of glucose in the intestines by functioning as inhibitors of glucosidase. Specifically, they target sodium glucose co-transporters and fructose transporters, such as SGLT1 and GLUT5, which are the primary transporters responsible for post-meal hyperglycemia in individuals with diabetes 27. The bitter melon extract contains quercetin, which has the ability to inhibit GLUT2. However, it does not have any noticeable impact on GLUT5 or SGLT1 28.
Intestinal sugar transport is also significant in causing high blood sugar levels after a meal. Diabetes patients exhibited elevated protein levels of the sodium glucose co-transporter SGLT1 and the fructose transporter GLUT5. These findings indicate that individuals with diabetes mellitus experience an enhanced absorption of carbohydrates in the intestines, which is linked to elevated blood sugar levels after meals. The ingestion of whole bitter melon juice decreases the absorption of glucose that is dependent on sodium (Na+) and potassium (K+) in rats with diabetes caused by STZ. The suppressive impact of bitter melon extract on sugar transportation may be more potent in circumstances characterized by elevated sugar transportation or hyperglycemia 29.
Histopathology of pancreatic tissue: Figure 2 presents a photomicrograph illustrating the histological features of the pancreatic. The Langerhans Islets in normal mice are easily discernible and exhibit well-defined borders (Figure 2A). The cells within the islets exhibit a well-organized structure, characterized by glands that are consistently sized and evenly distributed. The nucleus is round, with a clearly visible nucleolus, and the cytoplasm remains undamaged. Figure 2B displays a picture of the pancreas from a rat with diabetes under control. The image depicts clusters of tiny glands enveloped by columnar epithelium. The quantity of Langerhans islands it possesses is diminishing, and its structure seems to be deteriorating. The pancreas exhibited total atrophy and disintegration. Figure 2C depicts an image of the pancreas from a diabetic rat that was administered a DPP4 inhibitor. The findings indicated that the islets of Langerhans were in a favourable state and there was a reduction in pancreatic fibrosis. Figures 2D, E, and F depict diabetic mice that were administered ethanol extract of bitter melon fruit at doses of 75, 150 and 300 BW mg/kg, respectively. The findings indicated an augmentation in the size of Langerhans Islets and a reduction in pancreatic fibrosis. The treatment group administered with the ethanol extract of bitter melon fruit exhibited improved cell shape of the Islets of Langerhans in comparison to the diabetes control group.
The observed rise exhibited a dose-dependent trend, suggesting that MCE might possess the capacity to partially reinstate the functionality of organs with compromised insulin production. Moreover, prior investigations have shown the reparative properties of M. charantia and its pancreatic β-cell extract. These findings provide additional evidence for the efficacy of M. charantia extract in safeguarding and restoring pancreatic β cells 30. Furthermore, it was discovered that the portion of M. charantia containing a high amount of saponins successfully induced the release of insulin in MIN6 pancreatic β cells, with the extent of stimulation being depending on the concentration 31.
Conclusion :
The studies conducted on the effect of a dose of M. charantia ethanol extract as an anti-type 2 diabetes mellitus agent in Wistar rats induced by streptozotocin-nicotinamide have led to the following conclusions: The ethanol extract of bitter melon fruit (M. charantia) can elevate GLP1 levels in mice in a T2DM model. The ethanol extract of bitter melon fruit (M. charantia) can decrease dipeptidyl peptidase-4 in a mice model of T2DM. The ethanol extract of bitter melon fruit (M. charantia) exhibits a dosage-dependent reduction in alpha glucosidase levels in T2DM model mice. Specifically, at a dose of 300 mg/kg BW, the extract effectively reduces alpha glucosidase activity. However, this effect is not observed at other doses. The ethanol extract of bitter melon fruit (M. charantia) has effect to enhance glucose transporter 5 in a mouse model of T2DM. The shape of pancreatic tissue in a mouse model of T2DM is improved by the ethanol extract of bitter melon fruit (M. charantia).
Therefore, the outcomes of this study offer support for conducting additional research to confirm the effectiveness of bitter melon ethanol extract as a treatment for T2DM, and to use these results in broader clinical settings.
Ethical approval :
The protocol has obtained ethical approval from the Research Ethics Committee of the Faculty of Medicine at Sebelas Maret University, Surakarta. The approval number is 91/UN27.06.11/KEP/EC/2023.
Acknowledgement :
The Medical Pharmacology Laboratory of Muhammadiyah University and the Medical Research Laboratory at Jenderal Sudirman University, Purwokerto, Indonesia assisted the study.
Conflict of Interest :
The authors declare that there are no conflicts of interest.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
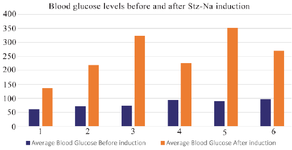
Figure 1. Graph of increase in blood glucose levels in each group before and after STZ-NA induction.
|
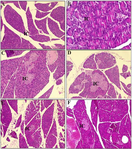
Figure 2. Results of HE staining of pancreatic tissue. A) normal control; B) STZ-NA induction; C) STZ-NA induction+DPP4; D) MCE 75 mg/kg BW; E) MCE 150 mg/kg BW; F) MCE 300 mg/kg BW. IC: islet cell.
|
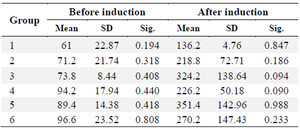
Table 1. Blood glucose measurements conducted prior to and following induction yielded the following results
|
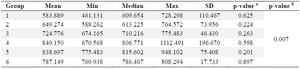
Table 2. Glucagon-like peptide-1 level
- a) Normality test, b) Homogenity test.
|
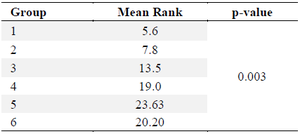
Table 3. Kruskal-Wallis test for GLP-1 levels
|
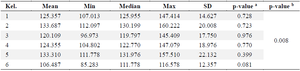
Table 4. Dipeptidyl peptidase-4 level
- a) Normality test, b) Homogenity test.
|
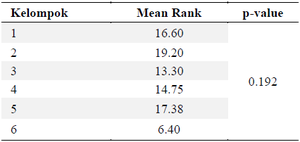
Table 5. Kruskal-Wallis test for DPP4 levels
|
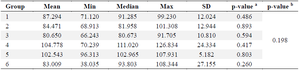
Table 6. Alpha glucosidase levels
- a) Normality test, b) Homogenity test.
|
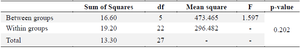
Table 7. Anova test for alpha glucosidase levels
|
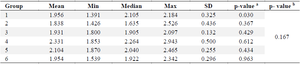
Table 8. GLUT5 levels
- a) Normality test, b) Homogenity test.
|
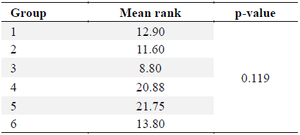
Table 9. Kruskal-Wallis test for GLUT5 levels
|
|