The Induction of L-lysine-α-Oxidase from Trichoderma Harzianum Rifai by Metabolic Products of Brevibacterium sp. and the Improvement of Its Isolation and Purification Techniques
-
Smirnova, Irina
-
T.T. Berezov Department of Biochemistry, Institute of Medicine, Peoples’ Friendship University of Russia (RUDN University), 117198 Moscow, Russian Federation
-
 Neborak , Ekaterina
T.T. Berezov Department of Biochemistry, Institute of Medicine, Peoples’ Friendship University of Russia (RUDN University), 117198 Moscow, Russian Federation, Tel: + 7 495 4343505; E-mail: katevladis@mail.ru, neborak_ev@pfur.ru
Neborak , Ekaterina
T.T. Berezov Department of Biochemistry, Institute of Medicine, Peoples’ Friendship University of Russia (RUDN University), 117198 Moscow, Russian Federation, Tel: + 7 495 4343505; E-mail: katevladis@mail.ru, neborak_ev@pfur.ru
-
Shkinev, Valeriy
-
Vernadsky Institute of Geochemistry and Analytical Chemistry, 119991 Moscow, Russian Federation
-
Larichev, Victor
-
The Gamaleya National Research Center of Epidemiology and Microbiology, Moscow 123098, Russian Federation
-
Shneyder, Yuri
-
Federal State Budgetary Institution All-Russian Plant Quarantine Center, 140150 Moscow, Russian Federation
-
Bashkirova, Ida
-
Federal State Budgetary Institution All-Russian Plant Quarantine Center, 140150 Moscow, Russian Federation
-
Karimova, Elena
-
Federal State Budgetary Institution All-Russian Plant Quarantine Center, 140150 Moscow, Russian Federation
-
Gavrilyuk, Lyudmila
-
T.T. Berezov Department of Biochemistry, Institute of Medicine, Peoples’ Friendship University of Russia (RUDN University), 117198 Moscow, Russian Federation
-
Ploskonos , Maria
-
Department of Fundamental Chemistry, Astrakhan State Medical University, Astrakhan 414000, Russia
Abstract: Background: The enzyme L-Lysine-α-oxidase from Trichoderma harzianum Rifai is a promising anticancer, antifungal and antibacterial agent. Intensive exploring of its physico-chemical properties and possible ways of application requires sufficient amounts of the protein which in turn depends on good techniques of cultivation of the micro-organism producer, enzyme soft isolation and purification "and storage".
Methods: An improved method has been suggested for isolation and purification of the enzyme. A specific combination of column sorbents was adapted and gradient elution with sodium chloride was applied to elevate the yield of the enzyme. The inductive influence of Metabolic Products (MP) of the Brevibacterium species, along with fungal metabolites of Ulocladium sp. and Trichoderma sp. was tested. The enzyme activity assay was based on the detection of oxidized dimethylbenzidine in a peroxidase reaction coupled with an L-lysine-α-oxidase reaction. Some enzyme properties were additionally explored.
Results: The upgraded technique of isolation and purification resulted in a yield of enzyme of about 79%. All strains of Brevibacterium sp. proved to be potent enhancers of L-lysine-α-oxidase activity and concomitant activities. The induced enzyme appeared to be less specific but more thermostable. Possible application scopes for the enzyme with modified properties are discussed. Phosphate buffer solution (pH=5.6) appeared to be the best one for long-term storage of the enzyme.
Conclusion: A significant inducing effect of MP of Brevibacterium sp. on L-lysine-α-oxidase has been detected, and its isolation and purification techniques have been improved.
Introduction :
L-lysine-α-oxidase (EC 1.4.3.2.) catalyses the reaction of oxidative deamination of lysine with the formation of a-keto-e-aminocaproic acid, NH3 and Н2О2 as products (Figure 1) 1. This microbial enzyme was first discovered in 1954 2 and isolated from Pseudomonas sp. Later it was found in Fungi, primarily in different species of Trichoderma sp., that appeared to be more abundant sources of this enzyme, than bacteria 3. The scientific interest in this enzyme is due to its anticancer, antibacterial and antiviral properties 1,4-10. The therapeutic potential and the necessity of deeper investigations generates the query for development of more rational methods of its production by biotechnological fermentation, isolation and storage.
Chronologically the first scheme of isolation and purification of fungal L-lysine-α-oxidase from Trichoderma was developed by Japanese investigators Kusakabe et al in 1980 3. The enzyme was obtained from a Trichoderma viride (T. viride) culture extract after surface cultivation of the producer. Such a technique of a producer cultivation is known to be associated with invasion of the equipment with fungal spores, but these investigations revealed that the strain T. viride was hardly producing L-lysine-α-oxidase in deep cultivation and therefore this method was recognized as unsuitable for accumulation of the enzyme. However, those were the pioneer studies which not only succeeded on isolation and purification of L-lysine-α-oxidase, but also proved its anticancer activity 3.
The scheme of isolation and purification of the enzyme suggested by Kusakabe H et al included six stages: precipitation with ammonium sulphate, thermal inactivation, two stages of DEAE-cellulose chromatography, chromatography with DEAE-sephadex A-50 and Sephadex G-200. The enzyme with a specific activity of 66.15 U/mg was obtained with a yield of the homogeneous enzyme of 8%. The Department of Biochemistry of RUDN University has been exploring L-lysine-α-oxidase under the supervision of academician T.T. Berezov since the 1980s. A new producer strain of L-lysine-α-oxidase Trichoderma harzianum (T. harzianum) Rifai VKPM F-180 was found and the specific enzymatic activity assay was developed 11-14. A new method was proposed for isolating and purifying the enzyme that included four stages, namely the extraction and purification with ammonium sulphate precipitation (35-60%) followed by chromatography with DEAE-cellulose and DEAE-sephacel, and gel-filtration with ultragel As-A-34. The enzyme had a specific activity of 29.0 U/mg, with a homogenous L-lysine-α-oxidase yield of 22.4% 12. For several years, the biotechnological processing of the producer strain T. harzianum Rifai VKM F-180 has been studied in detail and new methods for determining the enzyme activity have been suggested 14-19. Another producer strain Trichoderma auroviride Rifai VKM F-4268D and biological properties of its L-lysine-α-oxidase were also investigated 16,20-23.
The total amount of the target enzyme might also be increased by its induction with specific inducers. Currently, non-specific and specific inducers of biosynthesis of various metabolites are used with great success in various biotechnological processes 1,8. Sometimes inducers are synthesized chemically; however, biologically active substances widely spread in nature are often used for this purpose. Amino acids, vitamins, cofactors, and some rare substances of protein and lipid nature are often used in the search for more efficient technologies 1,8. Microbial secondary Metabolic Products (MP) are highly biologically active and are intensively investigated as a means of influence on other micro-organisms regarding biocontrol purposes or beneficial symbiotic effects as they can serve as inducers of the desired products 24,25. Investigation of microbial consortia that mutually affect each other is also a promising strategy as the costs of fundamental research of pure cultures are tens and hundreds of times higher than those of studying the industrial applications of microbial consortia 26. At the same time there is in fact no information in the literature on the use of MP of microbial origin as additives to nutrient media modulating the biosynthesis of L-lysine-α-oxidase.
The purpose of the current study was to investigate the possibility of increasing the yield of L-lysine-α-oxidase with the addition of sterile MP of bacterial and fungal origin to the producer strain and to optimize the procedure of L-lysine-α-oxidase isolation and purification. Substrate specificity and thermostability of the induced enzyme were assessed and the pH-optimum for long-term storage was identified.
Materials and Methods :
Fermentation and preparation of concentrate of culture liquid of T. harzianum Rifai F-180: The T. harzianum Rifai F-180 strain, obtained from the Federal Institution "State Research Institute of Genetics and Selection of Industrial Micro-organisms of the National Research Centre «Kurchatov Institute»", was used as a producer for L-lysine-α-oxidase and was cultivated according to the previously described technique 1,27. The fermentation was performed on the equipment of the Institute of Biochemistry and Physiology of Microorganisms of the Russian Academy of Sciences named after G.K. Skryabin (IBPM RAS). Briefly, fermentation was carried out on the BIOR-01 fermenter with a nominal volume of 100 L and a liquid filling factor of 60%. The fermenter was equipped with a magnetic stirrer, fine air filters, temperature and pH sensors. The fermentation medium was prepared directly in the apparatus. The device contained 60 L of purified water, 1% of wheat bran, 0.1% of adecanol stimulator and 1.3% ammonium sulphate, the pH value of 5.8-6.0 was set using 10% hydrochloric acid solution. Wheat brans were pre-soaked in 10 L of purified water for 4 hr, sterilized in an autoclave for 1 hr at 125°C, then were added into the bioreactor, where they were sterilized again along with other components.
The inoculum from flasks was added to the prepared bioreactor in a dose of at least 5% by volume. Cultivation was carried out at the temperature of 26°C, air consumption was 30 L/min throughout the fermentation, and the rotation speed of the agitator was 200 rpm. The pH value during growth was maintained at an average level of 6.5 (5.3-7.5). The duration of cultivation was 94-98 hr. At the end of fermentation, the cultural liquid was taken to the block of preliminary purification, where the mycelium of the fungus was separated by vacuum filtration on a Nutsche filter. The biomass of 3.5 kg was obtained, and the native solution was additionally centrifuged at 9000 rpm for 1 hr at the temperature of 2-4°C. The native solution in a volume of 55 L, obtained after removal of the biomass, was concentrated up to 1.5 L by membrane ultrafiltration providing the so-called semi-concentrate.
Enzyme isolation and purification: To determine the most efficient and gentle method of L-lysine-α-oxidase isolation different strategies and combinations of reagents have been tested. A stepwise protein precipitation with ammonium sulphate was applied initially for precipitation of impurity protein components and then for the precipitation of L-lysine-α-oxidase. The initial volume of the semi-concentrate was 300 ml, the concentrations of ammonium sulphate solutions were those of 15 and 70% for the first and for the second step, respectively. After centrifuging the precipitate was dissolved in a minimal volume of phosphate buffer solution (pH=7,4) and dialyzed against phosphate buffer solution (pH=7,4), containing 20 mM of sodium chloride. After that, two steps of column chromatography with DEAE-Sephacel (1.5 cm×20 cm column) and Sephadex G-100 (0.9 cm×90 cm column) were performed, the columns were equilibrated with phosphate buffer solution (pH=7.4). The elution was performed with sodium chloride in a gradient concentration of 0-0.8 M. All the procedures were performed at a temperature of 4°C. The specific enzyme activity assays were performed at each step of the procedure. Electrophoresis in 10% PAGE in the presence of 1% of sodium dodecyl sulphate and 1% of 2-mercaptoethanol was carried out to assess the purity degree of the isolated enzyme as described previously 1,8. The finally obtained concentrate of L-lysine-α-oxidase was exposed to lyophilization under vacuum and low temperature. The concentrate and lyophilized enzyme were then used separately for further experiments.
Additives of bacterial and fungal origin influencing the activity and substrate specificity of L-lysine-α-oxidase: Sterile cultural liquids of some bacteria and fungi strains, containing their MP, were added to T. harzianum Rifai F-180 in a volume of 1% of the fermentation medium on the first day and co-incubated for eight days of fermentation. Specifically, these were cultural liquids of bacterial amino acid producers, namely Lysine producer strain Brevibacterium sp. NITIA-86 28, Leucine producer strain Brevibacterium flavum 32-D 29, Proline producer strain Brevibacterium flavum АР-111, obtained from SPC Armbiotechnology 30, and fungi strains of Ulocladium sp., и Trichoderma sp., obtained from the All-Russian Collection of Micro-organisms (VKM). The fermentation, isolation and enzyme activity assay were then performed according to the above-described scheme. The direct influence of bacterial MP on lyophilized L-lysine-α-oxidase was explored additionally to distinguish it from their inducing effect. Specifically, cultural liquid of Brevibacterium sp. NITIA-86 was added to the incubation mixture in a volume of 1% during the enzyme activity assay procedure.
Enzyme activity assay: The activity of L-lysine-α-oxidase was determined by the previously described methods 8,27 with the usage of horseradish peroxidase and dimethylbenzidine to detect the equimolar amount of hydrogen peroxide produced by L-lysine-α-oxidase at 450 nm. A set of amino acids (L-Lysine, L-Phenylalanine, L-Tyrosine, L-Arginine, L, D-Leucine, L-Histidine, L-Methionine, L-Cysteine, L-Tryptophan, L-Asparagine, L-Proline, L-Ornitine, Serva) were taken as substrates in the experiments.
Enzyme properties investigations: The thermostability of the obtained after fermentation with bacterial MP L-lysine-α-oxidase was investigated by incubating an aliquot of the concentrate of cultural liquid of T. harzianum Rifai during different time periods at a temperature of 80°C in a water bath and subsequent cooling and enzyme activity assaying. The purified L-lysine-α-oxidase isolated in the previous experiments 1 was divided into fractions that have been stored for 9 years in different buffer solutions, namely citrate buffer solution, acetate buffer solution and phosphate buffer solution. The buffer solutions were prepared by mixing specific acid and its sodium salt (citric and acetic buffers) or sodium phosphate dibasic and sodium phosphate monobasic salts according to standard methodics 31. These buffers were used for dilution of the concentrate (1:3 and 1:4) and further storage. Thus, the L-lysine-α-oxidase containing concentrate has been stored at different pH values at a temperature of 2-4°C and then the residual activity was quantified.
Results :
Improved scheme of the enzyme isolation and purification: As a result of the aforementioned multistage purification (Table 1), the concentrate enzyme with a specific activity of 292 U/mg of protein was obtained, which composed the yield of 78.6%. Electrophoresis in 10% PAGE showed that the purified enzyme was detected as a predominantly single band in the region of 60 kDa, which is consistent with previously reported data 1. After the final stage of the purification of L-lysine-α-oxidase, the enzyme was lyophilized from 11 ml of a solution containing 1.4 mg of protein with the activity of 410 U.
Studies have shown that an aqueous solution of the homogeneous enzyme L-lysine-α-oxidase is not stable. The lyophilized form of the enzyme is stable and retains its activity when stored at 4°C. The induction of L-lysine-α-oxidase activity and the extension of the spectrum of its specific amino acid oxidase activities of after cultivation in the presence of MP of bacterial and fungal origin. The L-lysine-α-oxidase activity was significantly induced by the MP of all the tested Brevibacterium species (Figure 2A), but not by the MP of fungal origin.
The substrate specificity of the control L-lysine-α-oxidase (obtained in the cultivation procedure without any bacterial or fungal MP) towards L-lysine is rather distinct and similar to that of T. viride origin 3 as has been described earlier 8,11,12 and can be seen from figure 2B. L-Lysine as the preferable substrate is degraded with the fastest speed (2.5 U/mg) and concomitant L-Phenylalanine-α-oxidase and L-Methion-ine-α-oxidase activities are less marked. So, L-phe-nylalanine-oxidase activity composes 9% relative to the L-lysine-α-oxidase activity while L-methionine-α-oxidase activity is observed in trace value (0.003 U/ mg). Bacterial MP appeared to be potent enhancers of amino oxidase activities for L-Lysine and for six other amino acids (L-Phenylalanine, L-Tyrosine, L-Arginine, D, L-Leucine, L-Methionine and L-Histidine), but not for L-Proline. In contrast, fungal MP did not induce quantitatively any amino oxidase activities, but caused an extension of the substrate specificity spectrum in a similar manner as bacterial MP.
MP of B. flavum 32-D, the known Leucine producer proved to be the most efficient inducer of L-lysine-α-oxidase activity. Specifically, it reached 12.2 U/mg which was nearly 5 times higher compared to the control value (Figure 2A). The concomitant amino oxidase activities were also significantly induced: L-phenylalanine-α-oxidase activity increased by 45 times, and L-methionine-α-oxidase activity increased by 800 times and reached 3.4 and 2.4 U/mg, respectively. The oxidase activities towards other aforementioned amino acids were elevated to nearly 30-50% of the L-lysine-α-oxidase activity (Figure 2B). MP of Brevibacterium sp. NITIA-86, the Lysine producer, increased the L-lysine-α-oxidase activity up to 8.3 U/mg (Figure 2A), which is 3,4 times higher compared to the control. The concomitant amino acid oxidase activities reached approximately 30-40% from this value (Figure 2B).
MP of B. flavum AP-111, the Pro producer, increased the L-lysine-α-oxidase activity 3.6-fold (Figure 2A), specifically up to 8.9 U/mg, while the increase in other amino acid oxidase activities manifested within the range of 9-35% of the main lysine-α-oxidase activity (Figure 2B). So, this biostimulator seems to be a potent inducer of the L-lysine-α-oxidase with the lowest extension of substrate specificity spectrum. The addition of sterile culture fluid of the MP of fungi Ulocladium sp. and Trichoderma sp., which are not producers of any amino acid in sufficient quantities, did not exert any visible stimulating effect on L-lysine-α-oxidase production different from the control, but resulted in a similar extension of substrate specificity spectrum like that in the case of MP of bacterial origin (Figure 2B).
The direct influence of MP of bacterial lysine producer Brevibacterium sp. NITIA-86 on the specific activity of lyophilized L-lysine-α-oxidase: There was no increase in oxidase activity towards L-lysine detected under the direct influence of the MP of Brevibacterium sp. NITIA-86, but the concomitant oxidase activities were slightly elevated towards L-Phenylalanine, L-Tyrosine, L-Arginine, L-Histidine, L-Methionine, L-Ornitine. Also, some trace activity towards L-Cysteine, L-Tryptophan, L-Asparagine, and L-Proline manifested, extending the initial oxidase spectrum (Figure 3). Taken together, these results show, that the observed increase of of L-lysine-α-oxidase activity after cultivation of the producer strain in the presence of MP is due to its induction, but not to a direct influence of MP on the enzyme.
Enzyme thermostability and the effect of pH on the stability of the enzyme concentrate during long-term storage: The effect of heating on the stability of T. harzianum Rifai F-180 culture liquid concentrate during long-term storage in different time ranges was explored in further experiments. The thermal stability of the concomitant amino oxidase activities of the concentrate during its heating was also explored (Figure 4).
The preliminary experiments with the control enzyme revealed that heating the concentrate at 60°C leads to almost 30% loss in L-lysine-α-oxidase activity in 25 min, while the induced enzyme is more stable and retains its full activity after three hours heating at the same temperature. Its incubation at 70°C led to a decrease in L-lysine-α-oxidase activity by 16% and L-histidine-α-oxidase activity by 17% only, while heating up at 75-80°C led to a complete loss of enzymatic activity within a few minutes. We continued the study considering these findings and conducted experiments on thermal inactivation by heating at 80°C (Figure 4).
As can be seen from the figure 5, incubation of the concentrate at 80°C led to a total loss of L-lysine-α-oxidase activity after 150 s. Oxidase activity towards L-methionine was completely missing after 90 s, and for L-phenylalanine and L-tyrosine after 60 s. Moreover, these types of activities were disappearing much faster than L-lysine-α-oxidase activity. It is interesting to note that the thermostability profile of L-Phenylalanine-oxidase and L-Tyrosine-oxidase activities were similar apparently due to structural similarity of these substrates.
The influence of long-term storage on substrate specificity of L-lysine-α-oxidase was preliminarily ex-plored. It revealed spectrum of amino oxidase activities similar to the previously established one: high oxidase activity towards L-lysine (100%), and very low oxidase activities towards L-phenylalanine (8%) and L-methionine (1.2%). No oxidase activity towards other L-amino acids was detected. At the same time the enzyme activity in a water solution appeared to be unstable. The effect of the pH on its enzymatic activity during long-term storage was studied. Normally L-lysine-α-oxidase has the optimum pH of 5.6-5.9 1. The enzyme obtained in previous studies in the form of a culture liquid concentrate has been stored in different buffer solutions for 9 years. As can be seen from figure 4, this optimum pH is at the same time the most suitable pH for the storage. If the pH value is more acidic (4.4) or more alkaline (8.0), the enzyme activity drops by 3 and 2 times, respectively. When testing various buffer systems (citrate, acetate, phosphate), the phosphate buffer appeared to be to be the most suitable for storage as the enzyme activity remained the highest in this buffer and constituted 0.36 U/mg of protein (Figure 4).
Discussion :
The prospects for the introduction of L-lysine-α-oxidase into practice are associated with the improvement and cheapening of the technology for obtaining the enzyme, using simpler methods of its isolation and purification along with an increase of the desired enzyme production with the use of biostimulators. The suggested method of isolation and purification of the target enzyme is efficient, rationale, inexpensive and it allowed to obtain the enzyme with an activity of 292 U/mg of protein and with a yield of almost 78%.
The results of the present study were compared with the literature data on L-lysine-α-oxidase obtained biotechnologically from different sources 3,6,23,32-34. As can be seen from the table 2, the yields and the specific activities of the enzymes described in the referenced literature sources are much lower than those reported in the current study. These isolations L-lysine-α-oxidase were performed with the usage of different technological schemes, different column sorbents, and different elution regimens. The gradient elution with different concentrations of sodium chloride was applied in the study of Arinbasarova et al, 23 Amano et al 33, and Costa et al 6 but its reported highest concentrations were 0.3 M or 0.5 which were much lower than that of applied by us in the current study (0.8 M). The salt gradient has been applied in protein purification procedures for a long time and is still considered to be an effective biotechnological approach 35. So, it may be supposed that the gradient salt elution with sufficient final salt concentration was the crucial factor for avoiding losses in enzyme activity, although additional analysis of other factors should be recommended for deeper understanding the cause-effect relationships in the aforementioned data.
A relatively new powerful analytical tool - response surface methodology–is being intensively introduced into biotechnological studies. It is based on statistical design and allows to assess the contribution of various factors to the system development 36-38. This approach helps to increase the yield of a target enzyme manifold though is rather labor-intensive. However, it was not applied in the current study which was largely based on our previous experimental findings. We explored carefully in previous studies the influence of multiple parameters on the final yield of the target enzyme, such as pH of the incubation medium, temperature, source of carbon, type of the producer strain, regimen of cultivation (deep or surface cultivation) and various biological additives, including gibberellic acid, β-indolylacetic acid, specific amino acids, etc. 1,11,17. The surface cultivation technique was more efficient in the general outcome of the product than that of deep cultivation, but less affordable as it required a prolongation of the cultivation cycle. The most favourable carbon source proved to be wheat brans at pH around 6,5. The combination of chromatographic sorbents and salt concentrations for the gradient elution have been carefully selected in the current study to improve the outcome of the target enzyme. Previously we reported about 40% induction of L-lysine-α-oxidase activity by free L-lysine in a concentration of 40 g/l, while other tested bio-stimulants such as β-indolylacetic acid, 6-benzyla-minopurine, and gibberellic acid, along with other free amino acids L-phenylalanine, and L-methionine, appeared to be weaker inducers than L-lysine 1. It was observed in the current investigation that the inducing effect of MP of Brevibacterium sp. NITIA-86 (L-lysine producer strain) was much stronger than that of free L-lysine, specifically more than 3-fold, suggesting that other components presenting in the cultural liquid might also contribute to the induction of L-lysine-α-oxidase or even produce a synergistic effect. This last factor means indefiniteness of the system as any bacterial culture liquid is complex in their contents. The isolation and testing of individual substances of Brevibacterium sp. culture liquid may give some useful information for rational design, but the variety of secondary bacterial MP may be huge and their combined action on the induction of L-lysine-α-oxidase may be hard to be calculated and verified experimentally. So, it may be concluded that the surface response methodology seems hardly applicable for the current study, but the realized experimental approach appeared to be rather beneficial and lead to a result comparable with that of modern computational techniques.
The results of the current study may also be interesting from the perspective of possible application. The extended substrate specificity of the induced enzyme may be favourable for therapeutic anticancer purposes. The basis for anticancer use of amino acid degrading enzymes is that the synthesis of some amino acids in tumour cells is lowered or totally missing. A recent review of Pokrovsky et al highlights the significance of enzymes, degrading lysine, methionine, tyrosine and phenylalanine 39. In this context a broader substrate specificity of L-lysine-α-oxidase, induced by Brevibacterium sp. may be associated with a higher anticancer efficiency due to simultaneous depletion of multiple amino acids which is similar to a synergistic action synergistic action of several enzymes but doesn't additionally strain the immune system.
Conclusion :
An improved method of L-lysine-α-oxidase isolation from the cultural liquid of T. harzianum Rifai has been developed in the current study. The proposed technology for obtaining the concentrate, significantly reduces the stages of isolation and purification. The addition of MP of Brevibacterium sp. potently induces the production of L-lysine-α-oxidase and alters its substrate specificity and thermostability, which might be advantageous for some issues of its application. A suitable pH for long-term storage has been identified. These upgraded biotechnological techniques may be used for efficient L-lysine-α-oxidase production and for further experiments.
Ethical approval :
The study has been approved by the Ethics Committee of the Peoples’ Friendship University of Russia named after Patrice Lumumba (RUDN University), ethical code number 3-9-2024.
Conflict of Interest :
The authors declare no conflict of interest.
Funding: The article has no financial support.
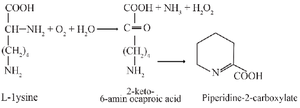
Figure 1. The reaction catalyzed by L-lysine-α-oxidase.
|

Figure 2. The induction of L-lysine-α-oxidase activity and the extension of its substrate specificity spectrum under the influence of bacterial PM. A) The induction of enzyme L-lysine-α-oxidase activity by MP of bacterial origin, U/mg of protein. B) The relative amino oxidase activities of the enzyme towards different amino acids, related to L-lysine-α-oxidase activity, %.
|
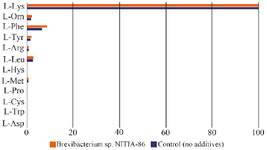
Figure 3. Relative amino oxidase activities of homogenous enzyme L-lysine-α-oxidase under the direct influence of bacterial stimulators, %. Slight increase in already documented concomitant L-amino oxidase activities is observed without any elevation of the key oxidase activity towards L-lysine.
|

Figure 4. The L-lysine-α-oxidase activity after 9-year storage period in different buffer systems in dilutions of 1:3 and 1:4. A) Citrate buffer solution. B) Acetate buffer solution. C) Phosphate buffer solution.
|
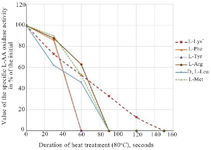
Figure 5. The influence of different durations of heat treatment at 80°C on the induced L-amino acid oxidase activities. Note: (*) Only those L-amino acids are listed for which the strain enzyme showed oxidase activity.
|
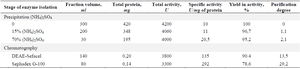
Table 1. The upgraded technology for isolation and purification of the enzyme L-lysine-α-oxidase from Trichoderma harzianum Rifai
|
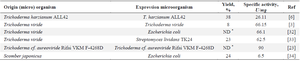
Table 2. The comparison of yields and specific activities of L-Lysine-α-oxidases isolated from different microorganisms
* ND means not determined or not presented in the article.
|
|