Therapeutic Effects of Nanochelating-Based Copper Nanoparticles on Burn Wound Healing in Mouse Model
-
 Rezvan, Hossein
Department of Pathobiology, Faculty of Veterinary Science, Bu-Ali Sina University, Hamedan, Iran, Tel: +81 34227350, +98 21 88992123; Fax: 81 34227475; E-mail: h.rezvan@basu.ac.ir
Rezvan, Hossein
Department of Pathobiology, Faculty of Veterinary Science, Bu-Ali Sina University, Hamedan, Iran, Tel: +81 34227350, +98 21 88992123; Fax: 81 34227475; E-mail: h.rezvan@basu.ac.ir
-
Zolhavarieh, Seyed Masoud
-
Department of Clinical Sciences, Faculty of Veterinary Science, Bu-Ali Sina University, Hamedan, Iran
-
Nourian, Alireza
-
Department of Pathobiology, Faculty of Veterinary Science, Bu-Ali Sina University, Hamedan, Iran
-
Bayat, Elham
-
Department of Pathobiology, Faculty of Veterinary Science, Bu-Ali Sina University, Hamedan, Iran
-
Kalanaky, Somayeh
-
Department of Research and Development, Sodour Ahrar Shargh Company, Tehran, Iran
-
Fakharzadeh, Saideh
-
Department of Research and Development, Sodour Ahrar Shargh Company, Tehran, Iran
-
Karimi, Pegah
-
Department of Research and Development, Sodour Ahrar Shargh Company, Tehran, Iran
-
Hafizi, Maryam
-
Department of Research and Development, Sodour Ahrar Shargh Company, Tehran, Iran
-
 Nazaran, Mohammad Hassan
Bachelor of Electrical Engineering (BEE) Official Member of American Chemical Society (ACS) and Owner of Nanochelating Technology (patented in USPTO, E-mail: mnazaran@nanochelatingtech-nology.com
Nazaran, Mohammad Hassan
Bachelor of Electrical Engineering (BEE) Official Member of American Chemical Society (ACS) and Owner of Nanochelating Technology (patented in USPTO, E-mail: mnazaran@nanochelatingtech-nology.com
-
Hamoon Navard , Sahar
-
Department of Pathobiology, Faculty of Veterinary Science, Bu-Ali Sina University, Hamedan, Iran
Abstract: Background: The aim of the present study was to investigate the potential of Nanochelating-based copper to accelerate the wound healing process and prevent infection in burn wounds.
Methods: Six to eight-week- old female BALB/c mice were burned with a 1 cm2 heated copper plate on the left flank and then divided into four treatment groups, treated with C8 (nanochelating-based CuNPs), cold cream (supplementary materials) as a control drug, Silver Sulfadiazine and no treatment, respectively. Skin tissue samples were taken from the mice on days 0, 3, 8, 15 and 24. One piece was fixed in 10% neutral buffered formalin for pathological examination and the others were stored at -80°C until used for pro-inflammatory and growth factor gene expression.
Results: The healing process in the group treated with 10 mg/ml C8 was significantly faster, and the survival rate of the mice in this group was significantly higher than in the other groups. The pro-inflammatory genes were expressed and down-regulated earlier in the C8 treated mice. Histopathology confirmed the higher cure rate in the group treated with 10 mg/ml C8 compared to other control groups.
Conclusion: C8 has beneficial effects on the healing of burn wounds and the effective dose of this compound should be further investigated. The present study demonstrates the anti-inflammatory properties of nano-chelate-based copper particles' on mouse skin burns. This research opens up new possibilities in dermatology and burn therapy and highlights the potential of copper-based formulations in the treatment of burn injuries.
Introduction :
Burns are one of the most common injuries characterized by severe skin damage resulting in cell death in the affected tissues; but depending on the cause of the burn, most people recover without serious consequences 1. The destruction of skin structures requires immediate medical care to ensure regeneration and restorative functions. Healing of cutaneous burns is a complex biological process involving four phases: hemostasis, inflammation, proliferation and tissue repair 2. In the hemostasis phase, platelets trigger chemical signals and activate the fibrin cascade in the blood vessels to form a clot that prevents bleeding and seals the injury 3. The inflammation phase consists of two overlapping steps; in the first step, phagocytic cells remove cellular debris in a process called phagocytosis 4. After burn trauma, the expression of proinflammatory and growth factor genes is severely impaired. In the late inflammatory phase (after 48-72 hr) the process of phagocytosis is continued by macrophages at the wound sites 5.
These cells also secrete tissue growth factors, in particular Platelet-Derived Growth Factor (PDGF), Transforming Growth Factor β1 (TGF-β1), Fibroblast Growth Factors (FGF), and collagenase, which are required for the activation of fibroblasts, endothelial cells, and keratinocytes. PDGF plays a crucial role in wound healing and its expression is essential for normal repair. However, excessive PDGF production may be associated with the formation of hypertrophic scars and keloids due to its stimulatory effect on fibroblasts and extracellular matrix production. TGF-β1 is immediately released in large quantities from platelets after injury. Neutrophils, macrophages, and fibroblasts active TGF-1 from platelets acts as a chemoattractant, and these cell types subsequently increase TGF-β1 levels in other cell types. Latent TGF-β1 is produced in the wound matrix alongside the active forms and enables prolonged release by proteolytic enzymes. During the healing process, proliferation phase, fibroblasts migration, collagen synthesis, angiogenesis, and tissue granulation this combination of multiple cellular sources and short-term storage maintains a steady supply of TGF-β1 6,7. Current treatment of burn wounds includes immunization of patients with tetanus prophylaxis and cleaning of the burn wound with 0.25% chlorhexidine, 0.1% cetrimide solution or any other mild, non-alcohol-based antiseptic 8.
Studies have shown that the introduction of copper into wound dressings would improve wound healing 9,10. Approaches are being considered for the benefits of copper particles based on nanochelating in the treatment of bure wounds. The use of Copper Nanoparticles (CuNPs) has been shown to stimulate the formation of new blood vessels and skin regeneration, thereby accelerating the healing of chronic wounds. The presence of copper oxide in wound dressings has been shown to promote angiogenesis and skin regeneration, contributing to the overall healing process. In addition, CuNPs support the release of growth factors that promote the anti-inflammatory healing of wounds, thereby significantly enhancing their antibacterial and antioxidant properties 11,12. CuNPs exhibit pronounced anti-inflammatory properties and heal burn wounds by reducing inflammation. Researchers have shown that copper ions can promote the mechanisms of skin regeneration and wound healing depending on their concentration. Wounds treated with CuNPs showed increased collagen content, accelerate re-epithelializ-ation, and increased capillary formation indicating anti-inflammatory and regenerative functions 13.
Nanotechnology has introduced particles of a variety of materials, typically ranging in size from 10-1000 nm 14. In recent years, nanomedicine and nanotechnology have been heavily utilized in burn infections and wound healing 15. This technology provides a range of engineered nanostructures that can be used for both therapeutic and diagnostic applications in burns 16. With the breakthrough of nanotechnology in various medical fields, several studies have reported the efficacy of CuNPs in wound healing 17,18. Nanochelating technology is a modern method to synthetize various nanostructures and previous studies have confirmed the efficiency of this technology in various medical field, e.g. in the treatment of chronic diseases 19-21, increasing the quality and quantity of stem cells 22, the antibacterial and antibiofilm activity of nanochelating-based silver nanoparticles (AgNPs) against various nosocomial pathogens 23 and other studies have also shown that AgNPs have antibacterial effects against Pseudomonas aeruginosa (P. aeruginosa) strains 24. Clinical data support the use of copper in the treatment of chronic wounds healing 11.
The clinical usefulness and efficacy of nano-copper solution is an antibacterial wound cleanser, accelerates healing of chronic wounds and has low toxicity 25,26. The use of CuNPs to accelerate the wound healing process, reduce inflammation, stimulate collagen production, and remodel tissue as well as their incorporation into scaffold materials for dressing to provide antibacterial and healing properties, represents a notable potential of copper nanostructures in the treatment of burn injuries. By adapting or further implementing copper nanostructure, various avenues can be explored in the field of dermatologic and burn therapy 13,27. The effects of CuNPs synthesized based on nanochelating technology on wound healing were measured for the first time in this study, and no article has yet been published on the effects of these nanoparticles on wound healing.
Author’s previous research has shown that pro-inflammatory genes (IL-1β and IFN-γ) are expressed in wound, spleen and neutrophils of mice with burn wounds, but Silver Sulfadiazine decreased the expression of pro-inflammatory genes and healed the wound by reducing inflammation 28. In the present study, C8 which is a nanochela-based copper nanoparticle (CuNP) was synthesized and its effects on improving the healing process of burn wounds was investigated.
Materials and Methods :
In the current study, a novel pharmacological preparation, derived from the application of nanochelating technology was administered to treat burn injuries characterized by full thickness. In addition, the effect of this nanoparticle was compared with that of Silver Sulfadiazine.
Animals: Female BALB/c mice aged 6-8 weeks were purchased from the Pasteur Institute of Iran. These mice were then housed in the animal facility of BU-Ali Sina University, in compliance with the established guidelines for animal care and ethical codes (code number: IR.BASU.REC.1398.049) in the animal house of Bu-Ali Sina University according to the animal study protocols. All animals were kept in a controlled environment (24±1°C and 12:12 hr light-dark cycle) and had free access to food and water. These guidelines were duly approved by the Research council of Bu-Ali Sina University, specifically for the purpose of inbred animal research. The mice in each group were euthanized and samples were collected.
Murine full-thickness burn & treatment: A number of 185 BALB/c mice were randomly divided into 7 groups; the first group consisted of 35 mice and the other 25 mice in each group. Burns were inflicted on the left flank of all mice in groups 1-4 using a 1 cm2 copper plate heated to a temperature of 200°C, with the temperature accurately measured using a thermometer. A volume of 100 µl Lidocaine 2% was then injected onto the left flank after shaving and the heated plate was placed on the shaved skin for 5 s without applying any pressure 29,30. All animals were kept under good conditions, with fresh water and freely accessible food. A pilot study was conducted to determine the dose prior to the main study. On this basis 4 doses were selected and included in the present study. After completion of the burn procedure, groups 1, 2, 3 and 4 were subjected to the following treatments: Group 1 was divided into 5 subgroups (A, B, C, D & E) with 7 mice and received different doses of C8 (nanochelating-based CuNPs 2, 4, 8 & 10 mg/ml) and one group was considered as drug control in this group, respectively. Lesions were treated twice daily (every 12 hr) by applying a mixture of C8 components at concentrations of 2, 4, 8, and 10 mg/ml together with cold cream (supplementary materials). After the effective dose of C8 was determined, group 2 received a control drug (Cold Cream as supplementary material), group 3 was treated with Silver Sulfadiazine, and group 4 received no treatment. On the other hand, groups 5-7, which were not exposed to burns, were treated as follows: Group 5 received C8 (nanochelating-based CuNPs), Group 6 received a control drug (cold cream as supplementary material), and Group 7 received no treatment. To perform the treatments, the entire surface of the wounds as well as an additional area of 5 mm around the lesions were covered with the drug twice daily 31,32 (Figure 1).
C8 (Nanochelating-based CuNPs) synthesis and characterization: C8 (nanochelating-based CuNPs) were synthesized by an advanced method for the preparation of chelate compounds based on US8288587B2 (Sodour Ahrar Shargh knowledge-based company-Tehran-Iran) and by a hydrothermal method. One g of copper carbonate (51% copper) was added as a reaction initiator in water-soluble organic acid under controlled temperature and pressure conditions. The solution was stirred for 2 hr and calcium hydroxide was added. After completion of the reaction, sodium alginate was added as an emulsifier and the calcium precipitate was separated from the solution. The calcium was filtered and the supernatant was dried in a spray dryer for 6 hr. Finally, the drug and cold cream (Saviz Company, Tehran, Iran) were completely homogenized at specific ratios. The surface morphology of C8 (nanochelating-based CuNPs) was characterized by Scanning electron microscopy (SEM: VEGA-TESCAN-LMU-LEO1455-VP; electron voltage: 10 kv model in Razi Metallurgical Research Center).
Evaluation of lesion size & sampling: The surfaces of the burn lesions were photographed using a professional Canon digital camera and the size of the lesions was calculated using the Image J software. Due to the inflammation caused by the burns and to prevent the inflammation from interfering with the main area of the wound, the size of the lesions was not measured on day 0. Imaging was performed for the groups from day 1 to day 33. Samples were collected from 5 mice in each group on days 0 (before the start of treatment), 3, 8, 15 and 24. Each sample was divided into two parts; the first part was fixed in 10% formalin for pathological examination and the other part was stored at -80°C until it was used for expression of pro-inflammatory and growth factor gene.
Primers: All samples were analysed with specific primers for the expression of the pro-inflammatory genes CCL4, CCL3, TNF-α, IL-1α, IL-12P35, IL-12P40, CCL5, CCR5, IL-1β and IFN-γ as well as the growth factors PDG, Keratinocyte Growth Factor (KGF), Epidermal Growth Factor (EPG). The sequence of the primers is listed in table 1.
RNA extraction: Total mRNA was extracted from the samples according to the protocol explained by Rezvan using the commercial DNAzist kit (DENAzist Asia, Iran) 12. Briefly, 100 mg of each lesion sample was placed in a tube and 1 ml of the lysis buffer (G1 buffer) was added, followed by 5 s sonication and a 5 min incubation at room temperature. The samples were then centrifuged at 12000 g for 15 min at 4°C and the aqueous part was transferred to a new tube. 200 µl of chloroform was added and vortexed for 15 s followed by incubation at room temperature for 3 min. The samples were then centrifuged at 12000 g for 15 min at 4°C and the upper phase was transferred to a new tube and mixed with an equal volume of isopropanol. An equal volume of the G2 buffer contained in the kit was added and mixed. The samples were then incubated for 10 min at room temperature and centrifuged at 10000 g for 10 min at 4°C. After discarding the supernatant and adding 1 ml of 70% ethanol, the tubes were quickly vortexed and centrifuged again at 10000 g for 5 min at 4°C. The supernatant was then discarded and 30-100 μl of nuclease-free water was added.
Construction of cDNA & Conventional PCR: The construction of cDNA from mRNA was performed using the commercial Sina-Clone/Iran kit according to the manufacturer instructions. 1 µl oligo-dT primer, 1 µl of dNTP and 10 µl of mRNA extracted from the samples were added to a tube. After incubation at 65°C for 5 min and a spinning at 12000 g for 2 min, the tubes were placed on ice and 2 µl of 10× buffer and 100 units of Reverse Transcriptase M-MuLV and 10 µl of distilled water were added. The samples were then incubated at 42°C for 60 min and then at 85°C for 5 min afterward.
For the conventional PCR 1 µl of cDNA, 0.25 µl Tag polymerase, 0.8 µl dNTP, 1.5 µl MgCl2, 5 µl 10× buffer, 37.5 µl H2O and 2 µl each of the forward and reverse primers were mixed and the PCR was programmed as 95°C for 10 min for denaturation, 32 cycles of 56°C for 45 s, 72°C for 50 s and 95°C for 45 s, and a final 72°C for 10 min.
Gel agarose electrophoresis PCR products were run on 1.5% (w/v) agarose gel and stained with 0.2 µg/ml DNA Safe Stain (Sigma Aldrich, USA, Country). The gel was visualized under ultraviolet light and photographed using a computer-assisted gel documentation (Canon, Japan).
Histopathology: Full-thickness skin samples, consisting of burned and normal areas were collected and fixed in 10% neutral buffered formalin. The tissue samples were then dehydrated, cleared, paraffin embedded, cut into 5 µm thickness, and stained with Hematoxylin & Eosin. The sections were examined independently by an experienced pathologist under a light microscope (CX41, Olympus, Japan) equipped with a digital camera (DP25, Olympus, Japan),
Pathologic and molecular examinations were performed on the samples collected up to day 24, and only the lesion size was measured as the main objective of the study on day 33. For sampling, the samples were collected from 5 mice in each group on days 0, 3, 8, 15 and 24. Imaging was performed for groups from day 1 until day 33 on remaining mice.
Statistical analysis: The results were statistically analyzed using the Excel and SPSS 22 softwares. One-way ANOVA test was followed by post hoc Tukey’s test were performed to analyze the results in all groups. p-value ≤0.05 as significant.
Results :
C8 (Nanochelating-based CuNPs) size: SEM image of C8 nanochelating based structure showed that the size of mentioned nanoparticles is about 50 nm (Figure 2).
Determination of the effective dose for C8 (Nanochelating-based CuNPs): Lesions were treated twice daily by applying a mixture of C8 components at concentrations of 2, 4, 8, and 10 mg/ml, with additional materials. The treatment was applied to the surface of the lesions and extended 5 mm around them.
Comparison of the effects of C8 and Silver Sulfadiazine on burn and healthy tissue: A dose of 10 mg/ml C8 cream was applied with supplementary materials (used as a control) to treat the burn lesions plus 5 mm around them twice daily. Com parison of lesion size in mice treated with C8 (at different doses, Figure 3A) and Silver Sulfadiazine, and controls drug (cold cream and no treatment) showed that the lesion size decreased significantly in the C8 group (10 mg/ml) compared to the other groups, except on days 3, 5, and 9 (p-value ≤0.05) (Figure 3B).
Different concentrations of C8 were tested as a pilot and the reduction in wound size with a dose of 10 mg/ml indicated a significant difference compared with the control group on days 3, 5, and 9 of the treatment (p-value ≤0.05). Concentrations of 4 and 8 mg/ml also showed useful therapeutic results at the end of treatment (Figure 3A) (p-value ≤0.05). After choosing the appropriate concentration of C8, different groups were treated with cold cream and Silver Sulfadiazine, as well as C8. The reduction in wound size in the Silver Sulfadiazine-treated groups was significant from day 17 until the end of the treatment, from day 9 to the end of treatment, the reduction in wound size was significant in the C8 group compared to the control group, but this reduction was significant compared with the untreated group from the beginning to the end of the treatment period. Silver Sulfadiazine also significantly reduced the size of the wound from day 9 compared to the untreated group (p-value ≤0.05) (Figure 3B). C8 nanochelating-based CuNPs have a positive effect on both burn healing and survival of the treated group compared to the Silver Sulfadiazine group.
Groups of BALB/c mice were burnt on the left flank with a 1 cm hot square plate at 200°C and treated with C8, Silver sulfadiazine, supplementary materials and no treatment (Figure 4).
Survival in burned mice treated with C8 and other control groups: The survival duration rate between the group treated with 10 mg/ml C8 compared to other control groups treated with Silver Sulfadiazine, cold cream and the group without treatment showed that the survival of mice were significantly more than other groups. Also the mortality rate was higher in the no treatment group (Figure 5) (p≤0.05).
Expression of pro-inflammatory genes: The expression of a number of pro-inflammatory and growth factor genes were examined in the burn wound treated groups using the conventional PCR method (Figure 6). The results clearly indicated different expression patterns for pro-inflammatory genes after application of different drugs. In mice treated with C8, the pro-inflammatory genes were expressed earlier and downregulated earlier than in other groups. In the groups treated with C8 (10 mg/ml), gene expression was delayed and downregulated on day 24 of treatment. In the control group or in the group without treatment, the expression levels of the pro-inflammatory genes were similar or even higher than in the group without treatment.
Histopathology: Histopathological evaluation showed that the healing process with signs of re-epithelialization and development of the epidermis was observed in the group treated with C8 on day 24, but there was no significant difference in wound healing with Silver Sulfadiazine and the compound (C8) on days 0, 3, 8, and 15 (Figure 7). On the third day, the morphology of the wound in all groups showed destruction of the epidermis and severe inflammation, and no difference was observed between them. Mild edema was observed in the treatment groups with ointment and C8, while severe edema was seen in the untreated group on day 8. On the 15th day of treatment, re-epithelialization was observed in the treatment groups with ointment and sulfadiazine. Acute inflammation and edema occurred after the use of ointment, but epidermal repair was remarkable in the C8 treatment group. On day 20, in addition to re-epithelialization, infiltration of cells and granulation tissue were visible in the ointment and Sulfadiazine-treated groups, but granulation tissue was less developed in the C8 group (Figure 7).
Coagulum (asterisk), inflammatory cells infiltration (square), granulation tissue (circle), epidermal layer (large arrow), microvascular congestion (small arrow) and epidermal ridges (arrowhead)
Magnification = 100×, scale bar = 200 µm, Hematoxylin and Eosin.
Discussion :
Nanomedicine has the potential to improve the treatment of various diseases from incurable to those with a poor prognosis. Nanostructures were initially used as carriers for drug targeting and delivery, but today nanomaterials themselves have also been shown to have therapeutic benefits 33. Recently, nanotechnology-based methods have been able to accelerate the wound healing process through the use of nano-sized materials, enabling new approaches in the field of wound healing and prevention of the development of various skin diseases 34,35. Among nanoparticles, cop-
per plays an important role in various cells, it modulates various mechanisms of function of growth factors and cytokines which are involved in all phases of the wound healing process. In addition, copper plays an essential role in angiogenesis and regeneration of the skin and improves the healing process by inducing various important factors 11.
According to the latest report by the World Health Organization (WHO), an estimated 265,000 people die each year as a result of burn injuries, most of which are caused by infections. This is particularly the case in low-and middle-income countries, where survivors face a lifetime of morbidity 16. Various strategies have been used in the treatment of burn injuries. Infections from burn injuries limit surgical success and increase patient mortality. Therefore, the development of new drugs for the treatment of burns has always been a goal of research in the field of burn wound treatment. Copper is an essential trace element with anti-microbial and anti-inflammatory effects and involved in numerous processes that together constitute wound healing, including endothelial growth factor induction, angiogenesis, and the expression and stabilization of extracellular skin proteins 7,36,37.
The results were demonstrated as a positive effect for C8 (nanochelating-based CuNPs) healing on burn lesions. Healing progress between the groups treated with 10 mg/ml of C8 was significantly faster than other doses of the drug. In comparison with Silver Sulfadiazine, and other controls (supplementary materials and control), using C8 increased the healing rate in full thickness burn wounds and survival of burned mice. Reducing the wound infection can affect the healing process and the survival rate of the affected person. One of the most attractive therapeutic properties of copper is antibacterial effects. Paterson et al indicated that produced CuNPs proved antibacterial activity against Gram positive and Gram-negative bacterial species, both planktonic and biofilm bacteria in 2D cell monolayers, and in a 3D human model of infected skin 38. Also, it is shown that copper-containing structures can inhibit the development of Staphylococcus aureus (S. aureus) and P. aeruginosa bacteria biofilms 39, so it seems that the effect of C8 in improving survival rate is due to anti-microbial effects of copper ions.
Histopathology observations based on thickness of the new epidermal layer, thickness of granulation tissue, angiogenesis, compact of sebaceous gland-hair follicles and surface keratinisation also confirmed the higher healing rate in the group treated with 10 mg/ml of C8 compared with Silver Sulfadiazine and other controls (supplementary materials as cold cream). C8 induces the basal layer to form granulation tissue, which helps healing of burn or even other lesions. However, induction of granulation tissue was observed in healthy tissues, which needs to be more investigated.
In a model similar to the present study, use of copper compounds in the burn lesion reduced the level of inflammation, exudation and collagen deposition leading to complete re-epithelialization on day 14 of treatment 8. The morphology of the burn wound at the beginning of the treatment (days 3 and 8) did not show significant changes (Figures 7-A-D, E-H), but after 15 days of the treatment, the healing process were different in the treatment groups with cold cream, Sulfadiazine and C8, compared to the no treatment group, the dermal repair to form a thin layer, along with formation of skin accessories was remarkable in the group treated with C8 (Figure 7L). Re-epithelialization and development of epidermis with near to normal thickness and development of slightly thick epidermis was observed in day 24 of treatment in group treated with Sulfadiazine and C8 (Figure 7O & P). Survival curve showed that the life time in C8 treated mice was more than other groups (Figure 5) and these mice were more resistant against dehydration and infection.
Angiogenesis due to migration, growth, and differentiation of endothelial cells plays an essential role in the process of wound healing. Copper is known as an angiogenic factor which can regulate induction of Vascular Endothelial Growth Factor (VEGF) and Hypoxia-Induced Factor-1-alpha (HIF-1α) pathways. This element is an important mineral that is involved in several physiological and metabolic processes 11 by stimulating VEGF (growth factor) production, increasing the expression of integrins 11,40, and extracellular skin protein stabilization 36. Studies have shown that the interaction of angiogenin and copper is a new medication to treat chronic non-healing wounds 39,41-43. Alizadeh S et al indicated that CuNPs at 1 μM concentration is a potential NP for wound healing usage with no negative side effects 27. Cucci LM et al demonstrated that copper nanoparticle can stimulate interleukin-2 production, which leads to tissues repairement and wound healing through macrophages 41 due to Th cells function. Thus, it can be used to help tissue regeneration and repair damaged tissue 44-47.
The usefulness of CuNPs was investigated in systematic study in the healing of chronic wounds. It implies that CuNPs may have exceptional wound-healing capabilities by stimulating the release of growth factors that support the wound anti-inflammatory process by enhancing antibacterial and antioxidant activities 48. Down-regulation the expression of pro-inflammatory genes was observed in mice treated with C8 group compared to other groups treated with either Silver Sulfodiazin or cold cream. Several human disorders are influenced by the CCL5/CCR5 axis, which may be targeted for treatment, and studies have demonstrated that blocking the CCL5/CCR5 axis can reduce levels of inflammatory cytokines including TNF-α, IL-6, and CCL5 and reduction of symptoms in patients. Several inflammatory conditions are associated with aberrant CCL5/CCR5 interaction including: atherosclerosis, microglia inflammation, viral infections, and several malignancies 49,50.
The expression of growth factors including (EPG, KGF and PDG) was investigated in all groups. Growth factors were expressed only in the control and Silver Sulfadiazine treatment groups. In the groups treated with C8, the genes were not expressed; it seems that the improvement caused by C8 is not related to the expression of EPG, KGF and PDG. Increasing the concentration of anti-inflammatory cytokines, reducing inflammation and improving the wound healing process have also been observed in similar cases 8,51. The mechanism of copper compounds on the wound healing process includes increasing the expression of integrin, fibrinogen, fibronectin, fibroblast, TGF-β, TGF-α, VEGF, PDGF, collagen fibers (mainly type I and III), which leads to wound healing through mechanisms of homeostasis, inflammation, proliferation and regeneration 11. Therefore, in future studies, the effects of copper nanostructure should be evaluated as a topical ointment for faster wound healing after burns.
This research shows that the copper nanostructure accelerates the healing of burned skin after topical application, the reduction of cytokine expression shows that copper also has a strong anti-inflammatory effect without causing much damage. In the treatment of burn wounds, this study highlighted the potential of copper nanoarchitectures and their compatibility with aesthetic and dermatological applications 13. Copper play an important role in skin regeneration and collagen synthesis, both of which are essential for the wound healing process. Copper sulfide nanoparticles in an injectable hydrogel containing hyaluronic acid have shown the ability to promote the formation of new blood vessels and accelerate wound healing, highlighting their role in collagen synthesis and tissue regeneration 52.
CuNPs together with other treatment protocols are necessary to increase the efficiency in the treatment of burn wounds. Studies have emphasized the ability of CuNPs with other materials to increase their antimicrobial as well as therapeutic activity, and this result shows their compatibility in the field of combination drug therapies 12,13. Antibacterial and therapeutic properties of scaffolds can be increased by using CuNPs. The existence of nanomaterials in the structure of scaffolds as dressings such as copper nanostructures, have the potential to optimize the therapeutic effects of scaffold materials for burn wound treatment. These designs can improve skin regeneration, increase therapeutic activity, and regulate the release of therapeutic agents 13.
Conclusion :
In conclusion, CuNPs based on C8 nanoclathrate have a positive effect on burn healing compared to Silver Sulfadiazine which is a common drug used to treat burn wounds. Histopathological findings indicated that C8 have healing effects on burn wounds that caused epidermis with normal thickness tissue formation compared to conventional treatment.
Ethical approval :
Committee of Bu-Ali Sina University with the code of IR. BASU.REC.1398.049.
Acknowledgement :
This study was supported by Sodour Ahrar Shargh Company and Bu-Ali Sina University. The authors would like to thank Miss Sakineh Azami and Mr. Hossein Khoshrouzi for their assistance in the lab and animal work. All practices were approved in Bu-Ali Sina University by the ethics committee (ethical code Number: IR.BASU.REC.1398.049).
Conflict of Interest :
Mohammad Hassan Nazaran is the owner of Nanochelating Technology. The authors state that there are no competing interests.
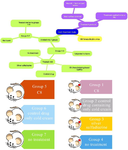
Figure 1. A scheme to explain the study design group 1-4 (cauterized), group 5-7 (without burning).
|

Figure 2. SEM image of CuNPs. CuNPs of approximately 50 nm are evenly disturbed throughout.
|
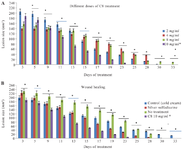
Figure 3. Lesion size treated with different doses of C8 (A) and treated with C810 mg/ml, Silver Sulfadiazine and control drug (B) at different times.
*p value ≤0.05 was significant.
|
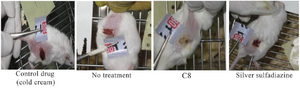
Figure 4. Burn wounds in BALB/c mice treated with C8, Silver Sulfadiazine, cold cream and no treatment.
|
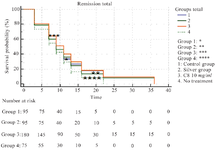
Figure 5. Comparison of survival duration in burnt mice treated with C8. *p-value ≤0.05.
|
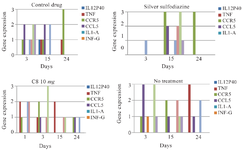
Figure 6. Expression of pro-inflammatory genes in in burn wounds treated with C8, Silver Sulfadiazine, cold cream and no treatment on days 0, 3, 15 and 24. The expression of genes is shown in three forms (no expression, borderline, weak and strong) with the numbers zero, one, two and three in three times, respectively.
|
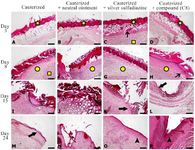
Figure 7. Photomicrographs of skin tissue samples obtained from burn wounds treated with C8 and Silver Sulfadiazine at different time points. A, B, C, D) Loss of epidermis and formation of coagulum on the wound surface along with underlying inflammation, edema, inflammatory cell infiltration and microvascular congestion. E, F, G, H) Granulation tissue formation, inflammatory cell infiltration, angiogenesis and microvascular congestion. I) Granulation tissue thickening and inflammation with no epidermal growth on the wound surface. J) Acute inflammation and edema of in the dermis with no sign of re-epithelialization on the wound surface. K: Granulation tissue maturation, reduced inflammation and epidermal layer reestablishment on the wound surface. L) Granulation tissue maturation, wound contraction, reduced inflammation and epidermal layer reestablishment. M) Granulation tissue maturation and partial re-epithelialization on the wound surface. N) Granulation tissue maturation and development of a thin epidermal layer on the surface with no skin appendages. O) Development of epidermis with near to normal thickness and newly formed epidermal ridges. P) Development of epidermis with normal thickness and epidermal ridges.
|

Table 1. Sequences of primers designed for pro-inflammatory genes and growth factors
|
|