Optimization of the Production of Soluble Recombinant TEV Protease in Two E. coli Strains
-
Shahriari , Matineh
-
Department of Pharmaceutical Biotechnology, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Tehran, Iran
-
 Shafiee, Fatemeh
School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran, Tel: +98 313 7927058; Fax: +98 313 6680011; E-mail: f_shafiee@pharm.mui.ac.ir
Shafiee, Fatemeh
School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran, Tel: +98 313 7927058; Fax: +98 313 6680011; E-mail: f_shafiee@pharm.mui.ac.ir
-
Bioinformatics Research Center, School of pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
-
Moazen, Fatemeh
-
Department of Pharmaceutical Biotechnology, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Tehran, Iran
-
Mir Mohammad Sadeghi, Hamid
-
Department of Pharmaceutical Biotechnology, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
Abstract: Background: The low solubility of Tobacco Etch Virus (TEV) protease, a functional enzyme that cleaves protein tags without significant modification in its sequence, is one of the most important limitations of this enzyme. In this study, the aim was to increase the solubility of TEV by changing the expression conditions and designing lysis buffer with various solubilizing agents to improve its solubility.
Methods: Escherichia coli (E. coli) BL21 (DE3) and E. coli origami harboring wild type TEV-pKR793 and mutant N23F TEV-pKR793 plasmids were used for the expression. Response surface methodology was used to determine the best culture conditions (IPTG concentration, incubation time and incubation temperature) of soluble expression. Furthermore, eight different solubilizing agents were added separately to the lysis buffer to check their effect on the protein solubility.
Results: The production of soluble N23F in E. coli BL21 (DE3) was two-folds more than the wild type and the inclusion body formation in the mentioned form was diminished as about 25% in comparison to the wild type. Finally, betaine had the most effects for enhancing the soluble expression of N23F in both host cells. For the wild type, sodium selenite, xylitol, and glycine showed the most effects on soluble production.
Conclusion: The solubility of the mutant form of TEV protease increased in E. coli BL21 (DE3) compared to its wild form. Also, using additives such as betaine to the lysis buffer, increased the solubility of N23F in E. coli BL21 (DE3) and origami strains.
Introduction :
Tobacco Etch Virus Protease (TEV) is a 27 kDa catalytic domain which can remove the fusion tags used to improve the solubility and stability of recombinant proteins 1,2. In addition to the usage for the cleavage of the purification tags, this enzyme is also used in studying protein-protein interactions in vivo, induced proteolysis and production of conditional mutations 3-5. TEV protease can be stable and active in a wide range of pH (between 4 and 9) 6,7 and temperatures (4-20°C) 1,2,8. Also, this protease can be expressed as an active and non-toxic protein in various host cells including Escherichia coli (E. coli), plant cells, yeast, insects, and even mammalian cells 9.
However, the limitations of this enzyme include enzyme self-cleavage which leads to less efficacy and also its low solubility (less than 1 mg/ml) which is another important drawback of this enzyme 1,10,11. So far, methods proposed to increase the solubility of TEV protease include change in expression conditions, co-expression with chaperones, the usage of solubility-increasing tags, changing the solvent conditions, site-directed mutagenesis, and changing the host cells expressing TEV protease 12-16. Although the mentioned strategies increased the solubility of TEV protease, the amount of produced protein in soluble form was much less than the insoluble protein.
In the previous study, a mutant form of TEV protease (N23F) was designed and expressed in E. coli BL21 (DE3) and it was shown that the soluble expression of the mentioned enzyme increased by 2.5 folds compared to its wild type according to computational and experimental analysis 17. On the other hand, addition of various reagents to the lysis buffer leads to the prevention in inclusion body formation during the cell lysis. It was reported that the use of 50 mM L-arginine and 50 mM L-glutamic acid significantly increased the solubility of several different proteins 18. In another study, the use of the same amino acid (0.1-1 M) facilitated the protein refolding 19. For TEV protease, in the previous study, various additives were used in the lysis buffer and their effect in producing the recombinant protein in soluble form was investigated. In the present study, a complex variable including the expression condition, the host cell strains, and solubility enhancers were tested for their probable positive effects on the soluble expression of wild type and N23F mutant in order to obtain an optimum condition to produce soluble TEV protease.
Materials and Methods :
Bacterial strains, plasmids and reagents: pKR793 vector (Addgene Co, Massachusetts, USA) containing the coding sequence of the wild type and mutant TEV protease was ordered to sub-clone by Biomatik (Canada). Bacterial strains were purchased from Pasteur Institute of Iran (Tehran, Iran). All chemicals including ampicillin and various reagents used as solubility enhancer were obtained from Sigma (Germany).
Design of protein expression conditions: The experiments for determining the best culture conditions (incubation period, temperature of incubation, and the inducer concentration) were designed using the Box-Behnken model of Design Expert® software. Each variable was evaluated at three levels of -1 (lower value of the variable), 0 (central limit of the variable) and +1 (higher value of the variable) and 15 conditions for protein expression were proposed with triplicated experiments in the central point. Each of these conditions were examined for two E. coli strains, (BL21 (DE3) and Origami for the production of the wild type and mutant enzymes.
Protein expression: Twenty ml of Luria Broth (LB) culture medium containing ampicillin (100 µg/ml) was poured in four Erlenmeyer flasks. Then, a colony of transformed strains with recombinant pKR793-wTEV or pKR793-N23F was added to 5 ml of prepared medium and placed in a shaker incubator for 16 hr at 37°C. In the next day, 1 ml of each overnight culture was added to 20 ml of fresh medium and incubated at the same temperature until it reached the exponential phase (OD 600 nm of 0.6). Then 15 expression conditions for each sample were examined. In each microcentrifuge tube, 1 ml of the relevant culture content was poured and then according to what was determined by the software, isopropyl β-d-1-thiogalactopyranoside (IPTG) was added with the determined concentrations to induce protein expression. Then the samples were placed in the incubator according to the temperature and duration determined by the software.
Comparison of the expression of the soluble and insoluble proteins in various culture conditions: In order to separate the soluble and insoluble proteins, 500 µl of Phosphate-Buffered Saline (PBS) was added to each pellet of various culture conditions and re-suspended and transferred to the microtubes containing glass beads (0.75 g). Then the microtubes were placed inside the microsmash device and centrifuged (1 min of centrifugation at 4500 rpm and 1 min of recovery on ice, repeated 5 times). Finally, the contents of microtubes were returned to the previous microtubes, and the microtubes were centrifuged at 7000 rpm for 5 min. At this stage, the supernatant (soluble sample) was removed and poured into new microtubes, and the sediment (insoluble sample) remained inside the microtube. Then, 200 µl of PBS was added to the microtubes containing sediment, and they were all placed in a -20°C freezer 1. SDS-PAGE was used in order to detect and determine the amount of the soluble and insoluble proteins. Also, GelAnalyzer software was used to determine the bands intensity and volume in comparison to the human serum albumin used in the known concentration as the standard. Finally, the optimum culture condition was used for maximum soluble expression of wTEV or N23F in two various host strains and the final amounts of produced proteins were calculated as described previously 1.
Investigation the solubilizing effects of various additives in lysis buffer: After determining the best culture condition for soluble expression of wTEV or N23F, 1 ml of lysis buffer (2 mM tris-HCl pH=8, 500 mM NaCl, 10% glycerol, 0.025% sodium azide, and 10 mM MgCl2), various additives including trehalose (1.28 g), glycine (0.75 g), betaine (1.17 g), mannitol (0.45 g), proline (0.057 g), xylitol (0.76 g), sodium selenite (0.0043 g), and CuCl2 (0.0033 g) were added in the aforementioned final concentrations. Cell lysis was performed in the designed lysis buffer instead of PBS by microsmash methods as described previously 1 and after the separation of soluble and insoluble samples, they were analyzed in SDS-PAGE in comparison to human serum albumin with known concentration.
Results :
The best culture condition for producing each recombinant TEV protease is illustrated in table 1. Furthermore, the most expression level (µg/ml) in the best culture condition is summarized in table 2. Based on the results of Box-Behnken model, BL21 (DE3) strain of E. coli produced more TEV either in inclusion body or soluble forms. However, the production of N23F in the aforementioned host cell was 2 folds higher than the wild type. On the other hand, the inclusion body formation in the mutant form was diminished about 25% in comparison to the wild type. On the other hand, for origami strain, although the expression of the protein in soluble form was lower than BL21 (DE3) strain, but, again the soluble protein in mutant form was increased in comparison to the wild form of TEV.
Correlation of the expressed wTEV concentration in soluble form when it was produced in E. coli BL21 (DE3) and the investigated variables was determined using the Design Expert software and was represented by the following equation:
Y=7.44+1.33A+7.89B+0.84C+0.79AB+2.38AC-3.7BC
Where Y is the expression (μg/ml), and A, B, and C are temperature (°C), post‑induction time (hr) and inducer concentration (mM), respectively.
When N23F was tried to express in E. coli BL21 (DE3), this equation was conducted by the software:
Y=10.664+1.64a+0.27b-1.65c
On the other hand, when Origami strain was used as the host cell, following equations were conducted for the soluble expression of wTEV and N23F, respectively.
Y=0.447+0.74A+0.25B-0.7C-0.96AB+0.6AC-0.96BC+1.06A2+1.79B2-0.03C2
Y=2.69+0.45A+1.36B-2.18C
Finally, after the addition of various reagents to the lysis buffer in order to enhance the soluble protein production, as shown in table 3, almost all additives enhanced the solubility of TEV; betain had the most effects for enhancing the soluble expression of N23F TEV in both host cells. For the wild type form, the sodium selenite, xylitol, and glycine showed the most effects on soluble production of TEV. On the other hand, CuCL2 led to less solubility of either wild or mutant forms. Figure 1 shows the SDS-PAGE of expressed proteins in soluble form when they produced in E. coli BL21 (DE3) or E. coli Origami after the addition of various solubilizing agents. Also, table 3 summarized the expression level in soluble form (µg/ml) when these various solubilizing agents were added to the lysis buffer.
Discussion :
One of the main problems of recombinant TEV protease production is its low solubility 1,10,11. Due to the high cost of this enzyme, its production in soluble form will bring high economic benefits. In this research, the aim was to increase the soluble production of the mutant form of TEV protease in two E. coli strains by examining the effect of independent variables (IPTG concentration, temperature and incubation time) on the dependent variable (the level of soluble expression of enzyme). For this purpose, experiments were designed using the Box-Behnken model.
In the previous study, N23F mutant was expressed in E. coli BL21 (DE3) and it was observed that the solubility of this enzyme increased compared to the wild type form 17. In the present study, it was also observed that the mutant form of the enzyme expressed in BL21 (DE3) strain had more solubility than its wild type form.
Based on the changes made in the genome of the E. coli origami strain, refolding and enhanced solubility of recombinant proteins, especially those with disulfide bonds can be improved. However, there is no disulfide bond in TEV three dimensional structure and the results showed that changing the host strain from BL21 (DE3) to origami did not have positive effects on soluble expression levels of this enzyme in the wild type or mutant forms. Meanwhile, it seems that the mutant TEV in E. coli origami bacteria has more solubility than the wild form. One of the reasons for this increased solubility is that this mutated protease has a higher number of hydrogen bonds between the protein and the solvent (water) compared to the wild type, resulting in the higher water coverage of the protein leading to its enhanced solubility. Also, in N23F mutant, the polar residue of core region of TEV protease is replaced by non-polar residues. Therefore, it is expected that this mutation will increase the packing of the hydrophobic core of the protein and the stability of the enzyme 17. This can be important in N23F TEV mutation which had a small temperature stabilizing effect and slightly increased Tm as compared to the wild form; however, it was observed that the effect of the host cell strain in increasing its solubility is not as expected since the expression of the soluble mutant in E. coli origami was lower than its expression in E. coli BL21 (DE3), which is probably due to the lack of disulfide bond in this protease. But for some proteins, changing the host cell strain improved the solubility of the produced protein. For example, human tissue Plasminogen Activator (tPA), which belongs to the serine protease family is clinically used to treat thrombosis, when expressed in E. coli origami, its cytoplasmic production was increased 20.
It was shown in this study that the addition of some reagents to the lysis buffer can help protein solubility. The present results showed that the addition of betaine, proline and trehalose to the lysis buffer, had the greatest effect on increasing the solubility of the mutant form of the enzyme in E. coli origami. In one study, the solubility of several different proteins using L-arginine and L-glutamic acid was significantly increased 18. In another study, the use of arginine (0.1-1 M) facilitated the folding stage and increased protein solubility 19. On the other hand, for the wild type TEV protease, Mohammadian et al reported that L-proline, sodium selenite, and CuCl2 had the most effects in protein solubility 1, while in the present study, CuCl2 had negative effects on the solubility of the mutant form.
Conclusion :
The solubility of the mutant form of TEV protease increased in E. coli BL21 (DE3) compared to its wild type form. Also, using additives such as betaine to the lysis buffer, increased the solubility of the mutant form of this enzyme in E. coli BL21 (DE3) and origami strains. However, it is necessary to try to find other additives with optimal concentrations to increase the solubility of this enzyme.
Acknowledgement :
The content of this paper was extracted from the grant with number as 3401463, financially supported by Research Deputy of Isfahan University of Medical Sciences. This article does not contain any studies with human participants or animals performed by any of the authors. The Ethics Committee of Isfahan University of Medical Sciences approved this research with the code of IR.MUI.RESEARCH.REC.1401.245.
Conflict of Interest :
The authors declare no conflict of interest.
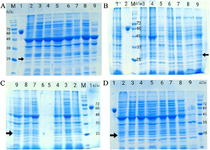
Figure 1. SDS-PAFE analysis of the addition of various solubilizing agents to the lysis buffer on soluble TEV protease production in E. coli BL21 (DE3) and Origami. A) N23F in Origami. Lane M: protein marker, lane 1: albumin, lane 2: trehalose, lane 3: betaine, lane 4: xylitol, lane 5: glycine, lane 6: manitol, lane 7: proline, lane 8: sodium selenit, lane 9: CuCl2. B) N23F in BL21 (DE3). Lane M: marker, lane 1: trehalose, lane 2: albumin, lane 3: bataine, lane 4: xylitol, lane 5: CuCl2, lane 6: manitol, lane 7: proline, lane 8: glycine, lane 9: sodium selenit. C) Wtev in Origami. Lane M: marker, lane 1: albumin, lane 2: trehalose, lane 3: bataine, lane 4: xylitol, lane 7: manitol, lane 8: proline, lane 9: glycine. D) wTEV in BL21 (DE3). Lane M; marker, lane 1: albumin, lane 2: trehalose, lane 3: bataine, lane 4: xylitol, lane 5: glycine, lane 6: manitol, lane 7: proline, lane 8: sodium selenit, lane 9: CuCl2.
|
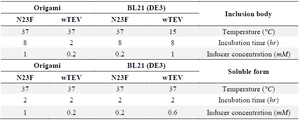
Table 1. The best culture condition for the soluble or inclusion body expression of wild and mutant forms of TEV protease in various host cells
|
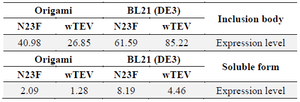
Table 2. The expression level of wild and mutant forms of TEV protease in various host cells in optimised culture conditions
Expression level is compared with albumin in the known concentration in µg/ml.
|
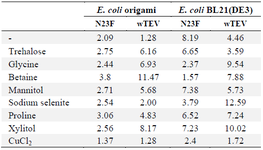
Table 3. The soluble expression level (µg/ml) of wild and mutant forms of TEV protease in various host cells in soluble form with the addition of various solubilizing agents
|
|