The Effect of Simulated Physiological Oocyte Maturation (SPOM) and L-Carnitine on Bovine Oocyte Developmental Competence
-
Malekpour, Ali
-
Department of Embryology and Andrology, Avicenna Research Institute, ACECR, Tehran, Iran
-
Sina Fanavaran Mandegar Company, Alborz Science and Technology Park, Kamalshahr, Iran
-
 Shirazi, Abolfazl
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 21 22432020; Fax: +98 21 22432021; E-mail: a.shirazi@avicenna.ac.ir, a.shirazi2023@gmail.com
Shirazi, Abolfazl
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 21 22432020; Fax: +98 21 22432021; E-mail: a.shirazi@avicenna.ac.ir, a.shirazi2023@gmail.com
-
Sina Fanavaran Mandegar Company, Alborz Science and Technology Park, Kamalshahr, Iran
-
Borjian Boroujeni, Sara
-
Department of Embryology and Andrology, Avicenna Research Institute, ACECR, Tehran, Iran
-
Sina Fanavaran Mandegar Company, Alborz Science and Technology Park, Kamalshahr, Iran
-
Sarvari, Ali
-
Department of Embryology and Andrology, Avicenna Research Institute, ACECR, Tehran, Iran
-
Sina Fanavaran Mandegar Company, Alborz Science and Technology Park, Kamalshahr, Iran
-
Naderi, Mohammad Mehdi
-
Department of Embryology and Andrology, Avicenna Research Institute, ACECR, Tehran, Iran
-
Sina Fanavaran Mandegar Company, Alborz Science and Technology Park, Kamalshahr, Iran
-
Pournourali , Mostafa
-
Department of Embryology and Andrology, Avicenna Research Institute, ACECR, Tehran, Iran
-
Sina Fanavaran Mandegar Company, Alborz Science and Technology Park, Kamalshahr, Iran
-
Behzadi, Bahareh
-
Department of Embryology and Andrology, Avicenna Research Institute, ACECR, Tehran, Iran
Abstract: Background: Simulated Physiological Oocyte Maturation (SPOM) mimics in vitro the physiological events of oocyte maturation in the presence of cAMP modulators. These modulators increase the intracellular concentrations of cAMP, which inhibits the immediate resumption of meiosis and gives the oocyte more time to gain optimal developmental competence. In addition, L-carnitine helps to increase the energy supply of cells through the β-oxidation of fatty acids. This study aimed to investigate the effect of SPOM and L-carnitine supplementation during In Vitro Maturation (IVM) and In Vitro Culture (IVC) on the developmental competence of bovine oocytes.
Methods: Ovarian Cumulus Complexes (COCs) were cultured in the presence or absence of forskolin+IBMX during the first 2 hr of IVM (pre-IVM) with or without L-carnitine (LC) during IVM or IVC in six experimental groups as follows: I) pre-IVM (pre-IVM group), II) pre-IVM with L-carnitine supplementation during IVM (pre-IVM/LC group), III) L-carnitine supplementation during IVM (IVM/LC group), IV) L-carnitine supplementation during in vitro culture (IVC/LC group), V) pre-IVM+ IVC/LC group, and VI) no treatment during IVM and IVC (Control group). The cleavage and blastocyst rates, the blastocysts’ total cells number, and the expression of Nanog, Bax, Oct4, Cdx2, and Ifnt genes in resulting blastocysts were assessed. To assess differences among experimental groups, a one-way analysis of variance was initially employed, followed by post hoc Fisher LSD. The difference between groups was considered statistically significant when p<0.05.
Results: The cleavage and blastocyst rates in the Pre-IVM and Pre-IVM/LC groups was higher than control group and other groups (p≤0.05) except for IVC/LC and IVM/LC groups, respectively. The number of blastocyst’s Inner Cell Mass (ICM) in pre-IVM and Pre-IVM/LC groups as well as the ratio of ICM/TE were higher than control group (p<0.05). The expression of OCT4, CDX2, and IFNT increased in both the pre-IVM and pre-IVM/LC groups compared to the control group (p<0.05).
Conclusion: In conclusion, the application of SPOM-adapted IVM and L-carnitine during IVM of bovine oocyte improves the quantity and quality of the resulting embryos.
Introduction :
Livestock play a pivotal role in rural livelihoods and are essential contributors to the economies of developing countries 1. In the last few decades, there has been an increasing focus on using assisted reproduction biotechnologies to improve livestock productivity 2. Ever since the first calf produced from In Vitro Embryo Production (IVEP) was introduced in 1981, significant progress has been made to enhance the quality of embryo production in bovines 3. Despite significant advancements in assisted reproduction biotechnologies, cattle In Vitro Production (IVP) still yields blastocyst rates of only 30-40% 4. This means that the quality of IVP-produced embryos is generally lower than that of in vivo counterparts, especially in terms of cryo-survivability and morphology 5.
During oocyte maturation, cells acquire the intrinsic competence to support development until the embryo can independently activate its embryonic genome. This is a complex process that is essential for successful embryo development 6,7. Successful development of bovine embryos depends on various factors, including the quality of oocytes and sperm, culture media, and the energy source 8. In the In Vitro Maturation (IVM) of the oocyte, there is a time gap between nuclear and cytoplasmic maturation, so that nuclear maturation starts earlier than cytoplasmic maturation. Cytoplasmic maturation of the oocyte, such as the accumulation of mRNA, proteins, redistribution of organelles, and cellular metabolic changes, along with nuclear maturation, are essential for the proper developmental competence of the oocyte. In mammals, cyclic adenosine monophosphate (cAMP) is necessary to maintain meiotic arrest in oocytes. During IVM, when the oocyte is removed from the antral follicle, it experiences a slight reduction in intra-oocyte cAMP concentration, which triggers the resumption of meiosis 9,10. There are approaches to delay nuclear maturation by increasing cAMP concentrations before or during IVM to provide an opportunity for cytoplasmic maturation leading to improved oocyte competence and embryonic development 11-13.
In this regard, various physiological and pharmaceutical methods have been used to inhibit the resumption of meiosis in cow oocyte. Physiological methods, such as cultivation in follicular fluid or cultivation in the presence of parietal granulosa cells are among these methods. Regarding pharmacological methods, substances that increase cAMP levels, such as Phosphodiesterase (PDE) inhibitors and Adenylate Cyclase (AC) activators, are effective in inhibiting the resumption of spontaneous meiosis in many animal species 14,15.
Forskolin (FSK) and 3-isobutyl-1-methylxanthine (IBMX) are small molecules known to elevate intracellular cAMP concentrations 16. FSK activates the enzyme AC, leading to an increase in intracellular cAMP levels. On the other hand, IBMX acts by blocking non-selective phosphodiesterase activity, preventing the inactivation of intracellular cAMP. This dual action of FSK and IBMX contributes to elevated cAMP concentrations within the cell 17-19. As previously mentioned, the modulation of cAMP levels during IVM plays a crucial role in enhancing the developmental competence of oocytes. Increasing the levels of this second messenger has been shown to significantly improve embryo development 20. Therefore, simulating the physiological maturation of the oocyte gives the oocytes more time to undergo appropriate cytoplasmic changes and increases the coordination of the maturation of the nucleus and cytoplasm, and on the other hand, provides the possibility of having a more homogeneous population of immature oocytes.
L-carnitine is a remarkable compound with several key functions. It serves as a potent antioxidant and is inherently involved in mitochondrial function and lipid metabolism by facilitating the transport of fatty acids to the mitochondria 21. Moreover, L-carnitine plays a vital role in fatty acid β-oxidation, contributing to increased energy supply to cell 22-24. Beyond its metabolic functions, L-carnitine is instrumental in regulating cellular processes, including apoptosis 25. It also serves as a guardian of cell membrane integrity and DNA, shielding them from damage caused by Reactive Oxygen Species (ROS).
To evaluate the effect of the presence of cAMP and L-carnitine modulators on the oocyte developmental competence, apart from evaluating the rate of cleavage and blastocyst formation as well as the allocation of blastocyst cells, the expression of some genes related to apoptosis, pluripotency and Maternal Recognition of Pregnancy (MRP) (3 and 43) were evaluated. The studied genes are: Bax as pro-apoptotic, NANOG and Oct-4 as markers of ICM and pluripotency, CDX2 as the primary initiator of trophectoderm formation and IFNT as an indicator of MRP that inhibits the expression of oxytocin and estrogen receptors. Considering the importance of synchrony between nuclear and cytoplasmic maturation, the aim of this study was to improve the developmental competence of oocytes matured in vitro 3. To achieve this goal, a protocol was implemented to investigate the possible effects of FSK and IBMX during pre-IVM as well as the effect of L-carnitine supplementation during IVM and In Vitro Culture (IVC) in bovine species.
Materials and Methods :
Experimental procedures in this study were approved by the Bioethics Committee at Avicenna Research Institute (approval no. 92014). Unless otherwise specified, chemicals were sourced from Sigma Chemical Company.
Oocyte collection and IVM: Upon arrival of slaughterhouse bovine ovaries, the ovaries were washed with normal saline containing antibiotics and any visible follicles with 2-8 mm diameter were aspirated with an 18-gauge needle attached to a 20-ml syringe. Cumulus-Oocyte Complexes (COCs) with more than three layers of cumulus cells and homogenous cytoplasm were selected for experimentation. The COCs were cultured in 50-μl droplets of maturation media consisting of bicarbonate buffered M199 (475 μg/ml), NaHCO3 (105 μg/ml), sodium pyruvate (2 μg/ml), penicillin-streptomycin (2.5 μg/ml), FSH (0.05 IU/ml), LH (0.01 IU/ml), and 10% Fetal Bovine Serum (FBS). During IVM, COCs were cultured for 24 hr at 38.5°C in an atmosphere of 5% CO2 in air with maximum humidity.
Pre IVM or Simulated Physiological Oocyte Maturation (SPOM): During Pre IVM, the COCs were cultured for 2 hr in the same culture condition as IVM in hepes buffered TCM-199 supplemented with 0.2 mg/ml Bovine Serum Albumin (BSA), 100 mM sodium pyruvate, 5 mg/ml recombinant human insulin/human transferrin (substantially iron-free), 10,000 IU penicillin, 10 mg/ml streptomycin, 100 mM forskolin, and 500 mM IBMX. The COCs were then cultured in IVM medium for 22 hr at the same culture condition as IVM.
In Vitro Fertilization (IVF): After maturation the COCs were subjected to IVF medium (10 oocytes/ 50 μl droplet) consisting F-TALP medium, supplemented with 0.2 mM sodium pyruvate, 5 mg/ml BSA (free fatty acid), 20 μg/ml heparin, and 5 μg/ml Penicillin-Streptomycin. The frozen-thawed bovine sperm was subjected to swim-down method using pure sperm gradient (500 µl 40% over 500 µl 80%), 750 g for 10 min. The motile sperm were added to IVF droplets at a final concentration of 2×106 spermatozoa/ml. The oocytes and sperm were co-incubated for 18 hr at 39°C with 5% CO2 in air and maximum humidity.
In vitro culture of embryos (IVC): After IVF, the presumptive zygotes were denuded by 3 min vortexing. The zygotes were then cultured in Synthetic Oviductal Fluid (SOF) supplemented with 2% (v/v) BME-essential amino acids, 1% (v/v) MEM-nonessential amino acids, 1 mM glutamine, and 8 mg/ml fatty acid-free BSA. The culture took place at a temperature of 39°C under conditions of 7% O2, 5% CO2, and 88% N2 in humidified air. On the third day of culture, 10% (v/v) charcoal-stripped FBS was added to the medium. The media osmolarity was maintained within the range of 270 to 285 mOsmol/L. The proportions of cleaved embryos, blastocysts, and hatched blastocysts were then determined on days 3, 6-8, and 9, respectively.
Embryo differential staining: To detect Trophectoderm (TE) and Inner Cell Mass (ICM) cells in blastocysts, double staining methods were performed. Briefly, to stain TE cells, blastocysts were exposed to 0.2% Triton X-100 for 20 seconds and then immediately immersed in 30 μg/ml Propidium Iodide (PI) for 1 min. For ICM staining, blastocysts were incubated in ethanol containing 10 μg/ml bisbenzimide (Hoechst 33342) for 15 min on ice. Stained blastocysts were mounted on glass slide and examined using an epifluorescence microscope. TE and ICM cells were counted by observing the red and blue colors of the cell nucleus, respectively.
Expression of Bax, Nanog, OCT4, CDX2, and IFNT genes: The expression levels of Bax, Nanog, OCT4, CDX2, and IFNT genes on day 8, blastocysts of Control, Pre IVM, and Pre IVM/LC groups were determined using quantitative RT-PCR (qRT-PCR). RNA extraction was carried out following the protocol outlined in a previous study 3. Subsequently, the concentration of RNA was measured with NanoDrop, and the isolated RNA was stored at -80°C. The Takara cDNA synthesis kit (Takara, Japan, Tokyo) was then utilized to synthesize from the extracted RNA as per the manufacturer's instructions. For relative qRT-PCR analysis, the housekeeping gene was employed as the normalizer gene. The sequences of the primers used for qRT-PCR can be found in table 1. qRT-PCR was conducted using the Step One Plus Real time PCR system (Applied biosystems, USA). Each reaction mixture compromised specific primers, synthesized cDNA, SYBR Green qPCR master mix (Takara, Japan, Tokyo) and distilled water, with reactions set up in triplicates for every run. Three independent biological replicates were performed. The relative qRT-PCR involved an initial denaturation step for 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, 30 s, and 30 s at 72°C. The results were analyzed using the Livak method (2-ΔΔCT formula) within the REST7 program.
Experimental groups: In this study, to evaluate the effect of FSK+IBMX and LC on oocyte’s developmental competence, the COCs (n=1009) were cultured in the presence or absence of Forskolin+IBMX during the first 2 hr of IVM (pre-IVM) with or without L-carnitine (LC) during IVM or IVC. Accordingly, the aspirated COCs were cultured in six experimental groups: I) in the presence of FSK+IBMX during the first 2 hr of IVM (pre-IVM; n=227), II) Pre-IVM with L-carnitine supplementation during IVM (pre-IVM/LC group; n=130), III) L-carnitine supplementation during IVM (IVM/LC group; n=287), IV) L-carnitine supplementation during IVC (IVC/LC group; n=89), V) pre-IVM + IVC/LC group (n=50), and VI) no supplementation during IVM and IVC (Control group; n=226). The study was followed with assessment of blastocysts cells allocation and the expression of Bax, Nanog, OCT4, CDX2, and IFNT genes in blastocysts of groups I and II, two groups that responded positively to the treatment, compared to the control group.
Statistical analysis: Data collection in this study was conducted across a minimum of four separate replicates. All collected data were presented as the mean value±SEM. To assess differences among the various experimental groups, a one-way analysis of variance (ANOVA) was initially performed, followed by post hoc Fisher LSD using SigmaPlot (version 11.0). In cases where the equal variance test was not met, treatments were compared using the Student-Newman-Keuls Method. Where the normality test failed, the Kruskal-Wallis ANOVA was employed. Differences between groups were deemed statistically significant when the p≤0.05.
Results :
Effects of SPOM-adapted IVM and L-carnitine on bovine embryo development: The cleavage rate in pre-IVM and Pre-IVM/LC groups was higher than the control and other groups (p<0.05) with exception of the IVC/LC group. The rate of blastocyst on the seventh day in the pre-IVM and pre-IVM/LC groups was higher than the control group and other groups (p<0.05) with exception of the IVM/LC group. There was no significant difference in the cleavage rate and blastocyst rate on the seventh day between the other groups and the control (Table 2).
Effects of SPOM-adapted IVM and L-carnitine on blastocyst cells number: The number of ICM and ratio of ICM/TE in the Pre-IVM and Pre-IVM/LC groups were higher than the control (Figure 1, Table 3; p<0.05).
Effects of SPOM-adapted IVM and L-carnitine on expression of OCT4, CDX2, IFNT, Nanog, and Bax genes: The expression of OCT4, CDX2, and IFNT genes increased in both Pre-IVM and Pre-IVM/LC groups (p<0.05). However, there was no significant difference pre-IVM group and control group in term of Nanog and Bax gene expression (Figure 2A). Also, the expression of OCT4 and IFNT genes increased in both Pre-IVM and Pre-IVM/LC groups (p<0.05). However, there was no significant difference between pre-IVM and control groups in term of Nanog, CDX2, and Bax gene expression (Figure 2B).
Discussion :
In addition to culture conditions, embryo quality is directly affected by oocyte developmental competence. The present study specifically investigated two issues in this context: the effect of Simulated Physiological Oocyte Maturation (SPOM) and L-carnitine on the ability of bovine oocytes to develop. These were selected for their potential to enhance oocyte quality during the IVM process. The results of the present study showed that the use of SPOM-adapted IVM system for bovine oocytes can significantly increase the cleavage rate on the third day compared to the control group. In addition, pre-IVM treatment had a significant effect on day 6 and day 7 blastocyst rates. Moreover, in the pre-IVM group, the number of ICM and the ratio of ICM/TE and ICM/total cells were higher than in the control group. As it is known, the improvement of IVM increases the efficiency of the embryo production process, and based on the studies conducted, the increase in oocyte cAMP levels during the IVM, increases oocyte competence 26.
In 2020, Leal et al 27 conducted a study on cattle to investigate the effectiveness of SPOM-adapted IVM system. They found that the SPOM-adapted IVM system had a positive effect on blastocyst production in cattle without a negative effect on embryo quality. Their findings showed that the SPOM could mimic the physiological events of maturation in vivo and could be a useful tool to improve oocyte competence. However, it is important to consider other factors such as the physiological state of the animal, the age of the sample donor, and changes in protocols when interpreting the results of previous studies 27. Overall, these findings provide valuable insights into the potential application of the SPOM in the field of reproductive biology 27. The results of the present study confirmed the finding of a previous study 28 and determined that SPOM-adapted IVM with FSK+IBMX increased the quantity and quality of blastocyst produced 28. However, these results differed from those reported by Razza et al 29, who reported that the use of the current version of the SPOM system may have adverse effects on oocytes and blastocysts and called for optimal protocols to improve oocyte competence.
Moreover, this study showed that the combined use of SPOM-adapted IVM and L-carnitine supplementation in IVM can increase the blastocyst rate on days 6 and 7, as well as the cleavage rate on day 3 compared to control group. Although L-carnitine supplementation in IVC could increase the cleavage and blastocyst rates on the sixth day as much as pre-IVM and pre-IVM/LC groups, but the total blastocyst rate on the seventh day in this group was lower than the two mentioned groups. Instead, when LC was added during IVM, the total blastocyst rate at day 7 was comparable to the pre-IVM and pre-IVM/LC groups (Table 1). On the other hand, the combination of pre-IVM and IVC/LC, did not have any positive effect on both cleavage and blastocyst rates (days 6 and 7) compared to the control group.
Previous studies show controversial results. In some studies, L-carnitine supplementation during IVM, IVF, and IVC increased the oocyte nuclear maturation rate, number of active mitochondria, cleavage, blastocyst, and hatched blastocyst rates 30,31, while in other studies, supplementation of L-carnitine during IVM, IVF, and IVC did not have any positive effect on the above items 31,32. For example, Phongnimitr et al 31 reported that while the use of L-carnitine during IVM improved oocyte nuclear maturation, it had no positive effect on development of vitrified oocyte and subsequent embryo development 32. Also, the addition of L-carnitine during IVM and IVC before embryo cryopreservation did not have a significant effect on the hatching rate after warming the frozen embryos 31. Despite these differences of opinion, the general agreement about the positive effect of LC in in vitro embryo production in different species is related to its positive effect as a potential antioxidant in reducing the accumulation of ROS and reducing apoptosis. On the other, the reduction of lipid droplets in the oocyte after the use of carnitine also increases the cryotolerance of the embryo 33,34.
In this study, although the cleavage and blastocyst rates were higher in the pre-IVM and Pre-IVM/LC groups than in other groups, L-carnitine supplementation in the pre-IVM/LC group could not further increase the above indicators. However, LC supplementation during IVM was able to improve total blastocysts rate to the same extent as the pre-IVM and Pre-IVM/LC groups. Moreover, the addition of LC during the IVC was able to increase the cleavage and day 6 blastocyst rate to the same extent as the pre-IVM and pre-IVM/LC groups (Table 1). In general, considering the accumulation of ROS in oocytes during IVM due to culture in vitro and oxidative stress, the use of L-carnitine is beneficial in reducing the amount of ROS during IVM 35-38.
The use of IBMX and Forskolin at the beginning of oocyte maturation increases the intracellular cAMP level and stops nuclear maturation and therefore maintaining the appropriate cAMP concentration in the oocyte is a vital requirement for the coordination of nuclear and cytoplasmic maturation processes during oocyte maturation 28,29. In this study, due to the specific effect of pre-IVM and to some extent L-carnitine on the developmental competence of the oocyte, the quality of blastocysts produced in both the pre-IVM and Pre-IVM/LC groups was higher than the control group, so that the ICM number and ICM/TE ratio were higher in both groups than control group (Table 2; p<0.05).
Regarding gene expression in the present study, OCT4 expression is essential for maintaining embryonic stem cells pluripotency and ICM functions, as well as for the expression of Nanog, Sox2 and other genes. It can modulate cell fate in the early stages of embryo development and plays an important role in embryo development 39,40. Nanog is also required to maintain the epiblast pluripotency and commitment in bovine embryo 41. The CDX2 gene regulates cell lineage specification during bovine early embryonic development, and IFNT is responsible for maternal recognition of pregnancy, implantation, and pregnancy maintenance (3 and 43). In this study, the higher cleavage rate and the quantitative and qualitative increase of produced blastocysts may be related to the significant increase in the expression of OCT4, CDX2 and IFNT genes in the pre-IVM and pre-IVM/LC groups compared to the control group.
The reason for the higher expression of OCT-4, CDX2 and IFNT genes in the pre-IVM and Pre-IVM/LC groups is at least partially due to the better culture conditions in these groups compared to the control group. The improvement of the culture conditions may be basically related to the presence of cAMP modulators; since the addition of L-carnitine to the IVM and IVC media could not improve the positive effect of cAMP modulators on the evaluated indicators, including the expression of OCT-4, CDX2, and IFNT genes.
Conclusion :
In summary, this study showed that SPOM-adapted IVM with or without L-carnitine significantly increased the developmental competence of bovine oocytes, which was associated with a quantitative and qualitative increase in blastocysts produced. This positive effect, apart from the better synchronization of nuclear and cytoplasmic maturation of the oocyte, may be at least partially related to the increased expression of pluripotency and implantation genes in blastocysts.
Conflict of Interest :
The authors declare no conflict of interest.
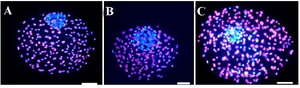
Figure 1. Epifluorescent microscopic imaging of bovine blastocysts produced by different approaches. ICM and TE nuclei were respectively stained with Hoechst 33342 (blue) and PI (red). A) Blastocyst/ Pre IVM, B) Blastocyst/ Pre IVM group (LC IVM), C) Blastocyst/control (scale bar: 30 μm).
ICM; Inner Cell Mass, TE; Trophectoderm, and PI; Propidium Iodide.
|
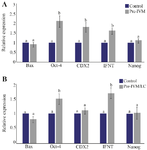
Figure 2. A) The expression of Bax, OCT4, CDX2, IFNT, and Nanog genes in Pre-IVM and Control groups. B) The expression of Bax, OCT4, CDX2, IFNT, and Nanog genes in Pre-IVM/LC and control groups. Numbers with different lowercase letters in the same column are significantly different (p<0.05).
|
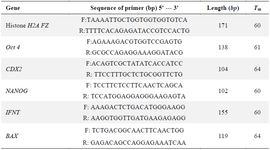
Table 1. The primers sequences used in Real Time PCR
|
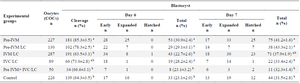
Table 2. The effect of pre-IVM and L-carnitine supplementation on bovine embryo development
a,b) Numbers with different lowercase superscript letters in the same column are significantly different (p<0.05).
|
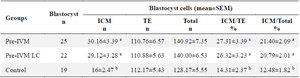
Table 3. Effects of Pre-IVM and Pre-IVM + LC on blastocyst cell number
a, b) Numbers with different lowercase letters in the same column are significantly different (p<0.05). ICM; Inner Cell Mass, TE; Trophectoderm.
|
|