Immunogenic Consideration of a Designed Polypeptide Against Brucellosis Compared to RB51: An In Vivo Study
-
Saadat, Mina
-
Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
 Bandehpour, Mojgan
Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences; Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Science, Tehran, Iran , Tel:+98 21 8866139; Email:m.bandehpour@sbmu.ac.ir, bandehpour@gmail.com
Bandehpour, Mojgan
Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences; Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Science, Tehran, Iran , Tel:+98 21 8866139; Email:m.bandehpour@sbmu.ac.ir, bandehpour@gmail.com
-
Department of Embryology and Andrology, Avicenna Research Institute, ACECR, Tehran, Iran
-
Kazemi, Bahram
-
Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Mosaffa, Nariman
-
Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Abstract: Background: Brucellosis in livestock and its transmission to humans through the consumption of contaminated dairy products is an important issue. The introduction of new approaches using immunogenic proteins against and diagnosing brucellosis is a serious issue in human health.
Methods: Brucella abortus contains five proteins including: MOXR family ATPase-α2, T9SS C-terminal target domain-containing protein, Cobyric acid synthase, Hypothetical protein, and VirB11 type IV Secretion protein, which were considered and the designed recombinant polypeptide was produced and evaluated. The pure recombinant protein ABOR with 549aa in combination with chitin as an adjuvant was injected subcutaneously into guinea pigs to evaluate their immunity responses.
Results: The results indicated that the ABOR recombinant protein induced Th1 immunity with high levels of specific IgG (IgG2a) as well as Interferon-γ (IFN-γ), Interleukin-2 (IL-2), IL-12, and Tumor Necrosis Factor-alpha (TNF-α), compared to the control group. Th1/Th2 ratio analysis demonstrated the efficacy of ABOR protein combined with chitin in stimulating cellular immunity in the animals.
Conclusion: Designed recombinant polypeptide combined with chitin showed ability for induction of cellular and humoral immunity an guinea pigs compared to RB51 vaccine.
Introduction :
Brucella species are facultative intracellular gram-negative bacteria categorized in the R-2 subgroup of the class proteobacteria, whose nomenclature is based on their pathogenicity and the reaction of the host immunity system 1. Brucellosisis a major worldwide zoonotic disease, causing infection in a broad spectrum of mammals, including humans. The annual incidence rate of brucellosis is about half a million people worldwide 2. Although cattle is the preferential host for Brucella abortus (B. abortus), it is one of the most infectious species of Brucella for humans 3. The subsequent distinct selection and characterization of the seroreactivity to different proteins of Brucella could lead to an acceptable diagnostic test and a subunit protein for prophylactic approaches. The characterization and identification of B. abortus-specific proteins reacting with circulating antibodies in naturally preferred infected cattle was performed in a previous study 4. This pathogen produces various virulent factors that modify phagocytic capacity, antigen presentation, cellular maturation, cytokine secretion, and apoptosis. Modulation of innate immune mechanisms by Brucella leads to the depression of T-cell immunity in chronic brucellosis patients 5.
Cytokines are the important biomarkers in immune system stimulation. The macrophages present Brucella antigenic structures to lymphocytes and produce Tumor Necrosis Factor-alpha (TNF-α) and Interleukin12 (IL-12). This modification enhance bactericidal activity of macrophages and cause differentiation of T helper cells to type 1 (Th1) leading to the control of the Brucella infection 6.
Vaccination of animals, especially in endemic areas, is the most economical and efficient way to control brucellosis, and minimizing potential human infections 7. Developing an effective preventive polypeptide for brucellosis has challenged scientists for many years. Only a few vaccines are commonly used to immunize cattle against B. abortus, including S19, RB51, 45/20, and SR82, with S19 and RB51 being the most widely used 8. Although the available vaccines effectively control brucellosis, they have numerous drawbacks, such as interfering with diagnostic tests, pathogenicity for humans, and the potential to cause abortion in pregnant animals, among others 9 virulent recurrence risk, secretion into milk and antibiotic resistance. Also, there are limitations to the immunization efficacy of RB51 depending on the challenge, with no absolute protection achieved with this vaccine 10. For these reasons, there is an urgent need for the development of an effective and safe subunit vaccine(s) for brucellosis.
Studies over the last decade have shown that multi-component vaccines (multi epitopes protein) induce more efficient responses than a single component subunit vaccine 10. Therefore, a more efficient immune response against Brucella infection could be achieved using a chimeric protein that includes several epitopes from different Brucella antigens.
Screening and evaluation of the bacterial antigens are the most important fundamental tasks in the development of subunit vaccines. While many proteins have been assessed as potential protective antigens against the Brucella infection, only a few have been shown to induce significant protection 11. The step one evaluation for degree of vaccine protection efficiency depends on the ability of the candidate antigens to direct immunity towards a Th1-type response 12. Also, to evaluate the selected antigen, a mixture of it and adjuvant, and delivery vehicle/vector is needed to trigger a strong immune response. For the development of an effective vaccine against intracellular pathogen such as Brucella, the production of Th1- derived cytokines [Interferon-γ (IFN-γ), Interleukin-12 (IL-12), Tumor Necrosis Factor-alpha (TNF-α)] as well as the activation of macrophages, dendritic cells, and CD4+ and CD8+T cells are the key factors; whereas Th2 immune responses, which are stimulated by the humoral immune system, have a less importance in the response to infection 13. In this study, the designed recombinant multi-epitope antigen named ABOR was expressed in Escherichia coli (E. coli) BL21 (DE3) and purified 4. It was then evaluated in combination with chitin as an confirmed adjuvant with several specific immunity system assays in guinea pigs as proper animal for veterinary vaccine investigations.
Materials and Methods :
Preparation of ABOR polypeptide: As in the previouse study 4 after identifying the bacterial determinant proteins, the ABOR polypeptide with 549aa was constructed with the epitopes from 5 selected proteins joined with GS linkers. The ABOR recombinant protein was expressed by synthesizing the protein-coding sequence with restriction enzyme sites (1710 bp) into the pET22b (5493 bp) expression vector (GeneCust, Luxembourg S.A.).
First, as the host cell, E. coli BL21 (DE3) was transformed with pETabor22b plasmid to express the recombinant multi-epitope protein. The prepared bacterial pellet containing recombinant ABOR protein was prepared and purified exactly similar to the earlier published method 4. Afterward, the purified ABOR polypeptide and the controls were lyophilized at -40 °C, complete vacuum for three hr.
Preparation of chitin microparticles as candidate adjuvant: To prepare the chitin microparticles in distilled water, a suspension of the pure chitin powder (C-7170, Sigma) was first sonicated and sequentially filtered using a 40-μm filter (Cell Strainer, BD Falcon, Mexico). Next, the chitin microparticles (<40 μm) were obtained by centrifugation at 2800×g for 10 min, drying at 40°C, and suspension in distilled water (100 μg/100 μl), then autoclaved and stored at 4°C before use. Then, particle size and size distribution were analyzed using a laser particle size analyzer (Malvern Master Sizer, Malvern Instruments, Ltd., Worcestershire, UK).
Lastly, the micro-particle and ABOR protein suspension were checked for the presence of endotoxin using a Limulus Amebocyte Lysate (LAL) kit (Cambrex). A 10-fold dilution series of the sample was prepared and equal volumes of the Limulus lysate were added to them. The test tubes were then incubated before being inverted and read. The mixtures remained unchanged and showed the sample did not contain Lipopolysaccharides (LPSs). It means all dilutions prepared from the protein ABOR compared to the kit positive control did not coagulate.
Analysis of the immunogenicity of the complex (ABOR+ chitin): The immune complex endotoxin negative formula contained 10 mg of ABOR protein and 50 µg chitin microparticles as an adjuvant for the first group. The other four groups were injected with 200 µL of Phosphate-Buffered Saline (PBS), 200 µl of RB51 vaccine (Lot number 2639, Colorado Serum Company), 50 µg chitin, and 10 mg recombinant protein, respectively.
Immunization of Cavia porcellus with ABOR protein: Fifteen 5-weeks-old Cavia porcellus (guinea pigs) weighing 300±20 g were purchased from the Pasteur Institute (Karaj, Iran) and divided into five groups: PBS, RB51 vaccine, ABOR protein, ABOR protein+ chitin, and chitin groups. Subcutaneous injection of 200 µl of injection formula was made into the back of the guinea pigs at three intervals of 10 days.
Sample collection and culture of lymphocytes: Blood samples of each experimental group were collected from a cardiac puncture before sacrificing the animals. The spleen of each animal was first dissected; next, the splenocytes were isolated by Ficol 1074 (Sigma) and then cultured in RPMI1640 (Biosera) (with 100 u/mL penicillin and 100 µg/mL streptomycin) at 37℃, 5% CO2, and 80% humidity in 5x106 cells for later immunological assay. All immunological tests were performed in triplicate. Additionally, liver tissue was removed and kept in formalin 10% until slide staining for the examination of any pathological changes following the use of the candidate immunogenic complex formula.
Assessment of the ABOR polypeptide specific IgG in sera of immunized guinea pigs by ELISA: The Enzyme-Linked Immune Sorbent Assay (ELISA) analysis was used to evaluate the immunogenicity of the injected polytope protein. Different concentrations of purified protein in 1×PBS were prepared and coated as described previously to set up the test. Briefly, the plate was first washed three times and blocked with blocking buffer after 1 hr at room temperature. Then, the plate was washed three more times, and 100 μl of diluted (1/500 and 1/200) serum samples previously prepared using 1×PBS was added to each well. The plate was left at room temperature for 2.5 hr. It was again washed three times, and 100 μl of HRP-conjugated anti-guinea pig IgG (1:10000 dilutions in 1×PBS) was added to each well and left at room temperature for 1 hr. After re-washing the plate, 100 μl of 3,3,5,5'-Tetramethylbenzidine (TMB) substrate (Sigma) was added and placed at room temperature for 15 min. After which, the reaction was stopped using 50 μl of 2N H2SO4. The Optical Density (OD) of each well was measured by an ELISA reader (TECAN, Denmark) at a wavelength of 450 nm with a reference wavelength of 630 nm. All of the 25 anti-Brucella IgG negative serum samples were measured to set a cutoff value using the mean±2SD.
Detection of the ABOR polypeptide specific IgG in infected cattle’s sera by ELISA: The ELISA method was used to investigate the binding of specific antibodies in cow's serum with brucellosis to ABOR recombinant protein because the selection of epitopes was from different proteins of the structural and secretory proteins of B. abortus. The method was performed as mentioned above; this time with cattle’s sera in a 1:1000 dilution. Healthy cow's serum was used to determine the cutoff value using the mean±2SD.
Expression assay of IL-2, IL-4, IL-5, IL-10, IL-12, TNF-α, and IFN-γ cytokine genes by Real-time RT-PCR: Total RNA was extracted from blood preserved with RNAlater solution using a Gene All Hybrid-R RNA Purification Kit (Seoul, South Korea) according to the manufacturer's guidelines. First, the RNA was then treated with 5 U of amplification grade DNase I (Fermentas, Lithuania) according to the manufacturer's specifications to remove any contaminating genomic DNA. Then, RNAs were quantified by measuring their optical density at 260 nm (OD260) using a Nanodrop-1000 spectrophotometer (Thermo Scientific), and the purity was assessed by determining the OD260/OD280 ratio, which was between 1.9 and 2. To synthesize cDNA, 2 μl of oligo (dT) primer and 2 μl of 2.5 mM stock of dNTPs (Fermentas, Lithuania) were mixed and added to 5 μl of DNase-treated RNA in DEPC-treated water, 4 μl of 5×M-MuLV buffer (Fermentas), and 1 μl of 5 IU/µl M-MuLV (Fermentas, Lithuania). The solution was incubated at 42°C for 1 hr. Next, the M-MuLV reverse transcriptase enzyme was heat-inactivated for 5 min at 72°C. Primers for all target sequences were designed using publicly available databases (GenBank) (Table 1). Then, a real-time PCR was performed with DNA Master SYBR Green I mix (Roche Applied Sciences, Mannheim, Germany) using 1 μl of the cDNA in a volume of 20 μl with the specific primers mix. After, a quantitative PCR was performed in the Step One TM instrument (Applied Biosystems) for 45 cycles with the following parameters: denaturation at 95°C for 15 s, annealing at 60°C for 10 s, and extension at 72°C for 15 s. Finally, the cytokines gene amplification was visualized by agarose gel electrophoresis.
To calculate the relative fold cytokine genes expression, the 2 –∆∆Ct method was used in comparison with the control group.
The lymphocytes proliferation assay by Flow cytometer: The proliferation of the five examined groups was performed by adding the antigens separately 24 hours after cell culture. Following six days of culture at 37°C in a 5% CO2 humidified incubator, the cells were harvested, and the proliferation rate was assessed by measuring Carboxyfluorescein Succinimidyl Ester (CFSE) uptake. After the CFSE-labeled (Life Technologies, USA) cells divide, their progeny will have half the number of labeled molecules. Each cell division was assessed flow cytometrically by measuring the corresponding decrease in cell fluorescence. In parallel, unstimulated lymphocytes were considered the negative control under the same culture conditions.
A stock solution of CFSE was prepared by dissolving CFSE (Molecular Probes, Eugene, OR, USA) in DMSO at a concentration of 5 mmol/L. In the labeling process, 1 ml cell suspension was added to the bottom of the falcon tube. Then, 1:10 PBS diluted CFSE stock solution was added at the transverse tube position, and the tube was rapidly turned and vortexed to ensure homogeneous dispersal. After labeling, the cells were incubated for 5 min in the dark at 20°C, after which 10×volume of RPMI was added and centrifuged again at 300 g for 5 min at 20°C. Next, the cells were washed three times, and 1 ml RPMI was added. Labeled cells were placed directly in culture media and incubated at 37°C in a 5% CO2 incubator for 72 hr in a 96-well plate to stimulate them to divide.
Toxicity evaluation of the ABOR protein: As a sensitive organ to the toxins, liver samples of guinea pigs were prepared with paraffin and then were cut for histological evaluation of ABOR protein on hepatic tissue. After Hematoxylin and Eosin staining, samples were studied.
Statistical analysis: All experiments were done in triplicate. Values were expressed as means±SD. Statistical analyses were performed by one-way analysis of variance (ANOVA) followed by Turkey’s multiple comparisons tests. p values less than 0.05 were considered to be statistically significant. REST@ 2009 Software (Qiagen, Hilden, Germany) was used to analyze RT-qPCR data.
Results :
Following previous study 4, a polypeptide extracted from antigenic epitopes of five proteins, MOXR family ATPase-α2, T9SS C-terminal Target domain-containing protein, Cobyric acid synthase, Hypothetical protein, and VirB11 type IV Secretion protein, was expressed in E. coli BL21. The designed poly-tope protein was called ABOR with 549aa, 47679.30 Da MW, and pI 5.23, with alanine (17% distribution) as the most abundant amino acid in the protein sequence. The confirmation steps were demonstrated below:
Confirmation of the ABOR recombinant protein production: In the previous study 4, western blot analysis of the protein expression revealed by His-tag antibody and infected cattle's serum showed that the ABOR protein with 549aa, 47679.30 Da MW, and pI 5.23 was successfully expressed after induction, and there were specific antibodies for their epitopes in the infected animal serum (results are available in the supplementary data).
Chitin micro-particles size analysis: In this study, chitin was used as an adjuvant. A laser particle analyzer determined the chitin microparticle size and size distribution. The particle size evaluation showed that 90% of the chitin microparticles in the suspension were less than 64.5 µ in size, 50% were less than 23.6 µ, and 10% were smaller than 6.01 µm (Figure 1).
Specific antibody to ABOR protein analysis in guinea pigs’ sera: Figure 2 showed that the guinea pigs immunized with ABOR protein+chitin or RB51 vaccine produced significantly higher IgG2a levels compared to the PBS control group.
The levels of IgG specific for recombinant ABOR protein in the group injected with ABOR+Chitin and whole B.abortus antigen (RB51) were determined by ELISA, with a mean absorbance values (A) at a wavelength of 450 nm of 2.051±0.217 and 1.864±0.115, respectively. The difference was not significant between immunized guinea pigs. In contrast, in the two groups of animals injected with ABOR+Chitin and RB51, the levels of IgG-specific increased significantly above those measured in the guinea pigs injected with PBS by a mean absorbance values (A) at a wavelength of 450 nm of 0.217±0.026 (p<0.0001). These results suggest that recombinant B. abortus protein (ABOR) combined with chitin is capable of responding similarly to the B.abortus vaccine in guinea pigs (Figure 2).
Identification of the interaction between the Cattle serum specific IgG and ABOR protein: The serums of healthy and infected cattle were gathered, and the amount of the produced IgG was determined by a manual ABOR protein-coated ELISA procedure. The results (Figure 3) show that IgG levels against ABOR recombinant protein were significantly higher in positive sera with a mean absorbance values (A) at a wavelength of 450 nm of 0.601±0.218 compared to negative sera with a mean absorbance values (A) at a wavelength of 450 nm of 0.218±0.078 (p<0.0001).
Comparison of cytokines genes expression levels: As shown in figure 4, real-time RT-PCR analysis of immunized guinea pigs’ spleen lymphocytes from the five guinea pigs groups receiving different immunization showed that expression levels of TNFα, IL-2, IL-12, and INF-γ significantly increased in guinea pigs immunized with ABOR protein+Chitin (p<0.05). In particular, an increase in the expression level of IL-12 and INF-γ in guinea pigs immunized by ABOR protein+Chitin was observed compared with guinea pigs immunized by RB51 (p<0.05). Moreover, a decrease in IL-10, IL-5, and IL-4 was also observed in this group compared to the controls (RB51, Chitin, and PBS, p<0.05).
The proliferation of Lymphocytes assay: Lymphocytes stimulation was assayed using flow cytometry to determine whether recombinant B. abortus ABOR protein elicited antibody responses in guinea pigs. The SI values of guinea pigs' spleen cells treated with B. abortus vaccine (RB51), recombinant protein (ABOR)+chitin, and PBS were identified on days 0 and 5 (Figure 5).
The Proliferation Index (PI) expressed the average OD value in the test group divided by the average OD value in the negative controls. As shown in figure 5, the mean values of the stimulation index in ABOR+chitin and RB51 groups were significantly higher than the PBS group on day 5 (p=0.0033 & 0.0021). Positive controls in the stimulation with RB51 showed the normal condition of cells in the culture. However, the ABOR+chitin group displayed a significantly different SI mean value (p=0.0235) compared to the RB51control group.
In addition, the ratio of the IFN-γ to IL-10, and IL-4 mRNA expression levels (Figure 6) showed the ABOR protein in complex with chitin was able to stimulate the production of IFN-γ, the key cytokine of cellular immunity that is produced by Th1 cells.
All desired ratios in each group were compared with other groups. In group ABOR protein+chitin, this ratio was significantly higher than the rest of the groups specially RB1. These results indicate the effective stimulation of gamma interferon production in group ABOR protein+chitin. In figure 6 the ratio between IFNγ and IL-10 in two sides of immunity is momentous in humoral and cellular stimulation after antigen injection. As well as these assays must be carried out in cattle exactly.
Microscopic evaluation: Histopathological evaluation revealed that mild portal and/or parenchymal hepatitis (mononuclear and polymorphonuclear cells), vacuolar degeneration, cell swelling, and individual necrotic cells were seen in all experimented groups, which were not related to treatment by the ABOR protein (Figure 7). Any necrosis or noticeable degeneration changes in hepatocytes were not observed.
Discussion :
Brucellosis is a zoonotic infection with a destructive economic effect on the livestock and public health in different regions 13. Therefore, vaccination of animals, especially in Brucella endemic countries, is the most efficient way to control brucellosis. This would also decrease the potential for severe human infections. However, live attenuated vaccine types, such as B. abortus RB51, are only partially effective in controlling animal brucellosis 14, and their disadvantages include abortion in some vaccinated pregnant animals, conflict with the diagnosis of vaccinated animals from an infected animal, resistance to antibiotics, and infection in humans 15. Therefore, there is a great need to develop a safer, and more effective vaccine against Brucella species. So, among vaccine types, subunit vaccines need to be introduced and developed to overcome these problems.
According to other studies, the Omp and ribosomal recombinant proteins from Brucella have been produced and shown to create efficient immunity in the mouse model 16. Furthermore, several studies showed that chimeric recombinant proteins could create a multilateral impact and increase the protective effect of each specific antigen 17. Therefore, it is believed that a multi-tope immunogenic protein containing immunodominant epitopes from several specific proteins may exert sufficient immunity against Brucella species.
One of the most important drawbacks associated with epitope-based vaccines is their low immunogenicity. To overcome this drawback, adjuvants are added to multi-epitope vaccines to improve the immunogenic constructs and modulate them toward the desired immune responses 18. Chitin is a potent activator of the innate immune response in macrophages 19. It can also elicit a type 2 immune response and augment the Th1, Th2, and humoral responses 20. In this study the particle size evaluation of the prepared chitins showed that 90% of the microparticles in the suspension were less than 64.5 µ in size, 50% were less than 23.6 µ, and 10% were smaller than 6.01 µm.
Phagocytosable small-sized chitin particles activated alveolar macrophages to express cytokines, such as IL-12, TNF-α, and IL-18, leading to INF-γ production mainly by Natural Killer (NK) cells 21. Pro-inflammatory cytokines produced at the onset of infection, such as TNF-α, and IL-12, have been shown to play a central role in the clearance of the bacterial organism in mice 22. So using the chitin nanoparticles in size of below than 64 µ as adjuvant was an idea in the present research. However, significant influence in INF-γ, IL-12, and IL-4 production results after injection of ABOR protein with chitin in comparison to ABOR protein without chitin was not observed.
The Th1 cytokine, IFN-γ, plays an important role in activating macrophages and limiting Brucella infections both in vitro and in vivo 23. However, the Th1 and Th2 cells are important regulators of the class of immune response. The role of the T-helper cells in amplifying immune responsiveness seems to be that Th1 cells are reliant on INF-γ and, to a lesser extent, IL-2 and IL-12. Th2 cells are heavily reliant on IL-4 and IL- 5 as well 24.
As a common control, the RB51 conventional vaccine. B. abortus RB51- an attenuated rough strain- was currently used as the vaccine of choice against bovine brucellosis in many countries 25. In this study, the mRNA expression levels of Th1 cytokines (IFN-γ and IL-2) increased in guinea pigs immunized with the ABOR protein in formulation with chitin. In addition, increased levels of TNF-α and IL-12 cytokines was observed. Polyepitope named ABOR protein successfully stimulated the cellular immunity system, as confirmed by the significant higher Th1/Th2 ratio compared with the control groups.
Other studies have demonstrated that the RB51 vaccine induces a strong Th1 cellular immune response with the production of IFN-γ and CD8+ specific cytotoxic cells in mice 26. The results of cytokine expression in guinea pigs immunized with the RB51 vaccine confirmed those results. Moreover, the Th1/Th2 ratio analysis data in the ABOR and ABOR and chitin groups demonstrated the efficacy of the protein in cellular immunity stimulation was significantly powerful. The results of cytokine genes expression showed that the ABOR protein in complex with chitin significantly induces IFN-γ and IL-12 production compared with the RB51vaccine (p<0.05).
An increased IL-10 mRNA expression level resulting from the repression of a protective Th1 response was observed in brucellosis, which supports the capacity of Brucella to avoid immune surveillance. In this study, the IL-10 expression level in guinea pigs immunized with ABOR protein and chitin decreased significantly (p<0.05). These findings are in agreement with Fu et al recombinant vaccine design research 27. Also, IL-4 is the typical priming cytokine, that drives the differentiation of naive T cells to Th2 pathway of immune activation. Th2 immunity ensures immunity against extracellulary pathogenic micro-organisms and main cells in the Th2 immunity are B cells, eosinophils and basophils. If the immunity is strongly biased to Th2 answer, a lot of IL-4 and IL-10, also IL-5 is produced and this can lead to allergic IgE mediated reaction or autoimmunity mediated by specific autoreactive antibodies 24. These cells further produce IL-4 and this way the Th2 immune pathway is enhanced. At the same time, IL-4 functions as the main contra regulatory cytokine that inhibits the shifting of immune response towards Th1 immunity. Therefore, IL-4 and IFN-gamma are contra regulatory cytokines. IFN-gamma is typical Th1 cytokine and IL-4 is typical Th2 cytokine. So, from the obtained ratios Th1 pathway is more enhanced as expected. As well as the results of IFN-gamma:IL-4 and IFN-gamma:IL-10 ratios show that Th1 cells produce a determined amount of IFN-γ that ensures the Th1 shifted immune pathway and at the same time function as the strongest contra regulatory cytokine against Th2 immunity, whereas, it prevents the development of Th2 immunity.
In the present research the opsonization pathway that is the most important protective role of antibodies in brucellosis was considered. Furthermore, a high concentration of IgG during infection of cattle appears to prevent complement-mediated extracellular bacterial lysis and promotes phagocytosis of Brucella, enhancing the extension of the disease 28. On the other hand, cell-mediated immunity plays a key role in a host’s defense against B. abortus, involving mainly activated antigen-presenting cells and CD4+, CD8+ T lymphocytes 29, 30.
Therefore, a multiepitope protein designed from several antigenic substances of the pathogen as a vaccine candidate and production of it by prokaryotic expression system was carried out. The guinea pig was selected as a proper laboratory animal for veterinary vaccinology study. Epitope-based polypeptides represent a new type of vaccine that specifically stimulate immunity against the selected epitopes, and as of now, Yin et al have been able to develop a multi-epitope subunit vaccine from Omp 2b, Omp 16, Omp 31, and BP 26 that conferred high levels of protection against B. melitensis in mice 18.
Altogether, guinea pigs immunized with the complex of ABOR protein and chitin or RB51 vaccine produced significantly higher specific IgG levels compared to the PBS group. This suggests that Th1-associated antibodies are generated in the humoral immune response against Brucella, enhancing the opsonization and phagocytosis activity. Furthermore, a comparison of PI in guinea pig spleen cells treated with B. abortus vaccine (RB51) and recombinant protein on days 0 and 5 suggested that ABOR protein and chitin is capable of responding in guinea pigs in the same manner as the B. abortus vaccine. Also, the levels of specific IgG against ABOR recombinant protein were significantly higher in positive sera with a mean absorbance values (A) at a wavelength of 450 nm of 0.601±0.218 compared to negative sera with a mean absorbance values (A) at a wavelength of 450 nm of 0.218±0.078 (p<0.0001) in the animals’ humoral system stimulated by ABOR polypeptide and chitin.
Conclusion :
In the present study, the 549aa designed recombinant poly peptide extracted from five structural or secreted proteins of B. abortus combined with chitin as adjuvant, was found to be important as an immunogenic complex without any allergic or toxic effect on liver tissue. It showed ability for induction of cellular and humoral immunity in guinea pigs compared to RB51 vaccine.
Ethical Statement :
The Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.AEC.1402.104) approved this animal research following the ethical guidelines of the principles of research on laboratory animals.
Acknowledgement :
The authors would like to thank the staff of the Cellular and Molecular Research Center at Shahid Beheshti University of Medical Sciences. This study was funded by the Cellular and Molecular Research Center at Shahid Beheshti University of Medical Sciences [grant number 43007351]. All authors have no conflict of interest.
Conflict of Interest :
The authors declare no conflict of interest.
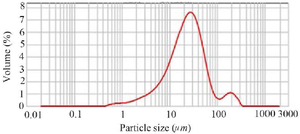
Figure 1. Chitin micropaticles size analyzing. More than 80% of the obtained microparticles were determined below 40 µm.
|
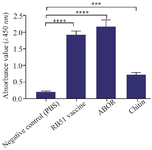
Figure 2. Specific IgG2a level in the serum of immunized guinea pigs. The comparison between specific immunizations of the animals injected with ABOR and Chitin, RB51 vaccine, and PBS.
|
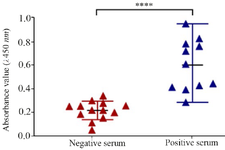
Figure 3. Specific total IgG levels measured by ELISA. Control (negative) serum and serum of cattle with brucellosis (positive) in interaction with ABOR protein.
|
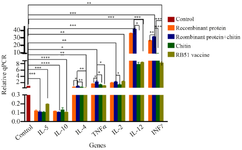
Figure 4. Expression levels of cytokine genes. Cytokine mRNA transcriptions levels after injection of ABOR recombinant protein and chitin, RB51 vaccine, Recombinant protein, Chitin, and PBS (control) were compared. (p<0.05). The 2 –∆∆Ct method was used. A higher IFN-gamma: IL-4 ratio (p=0.04) was found in ABOR protein+chitin group compared to controls. This group also displayed higher mRNA levels of IL-12 (p=0.03), but not IL-4 and IL-10. IFN-gamma expression in ABOR protein+chitin group, but not controls, correlated strongly with IL-12 level.
|
![<p>Figure 5. Comparison of Proliferation Index (PI) in guinea pigs' spleen cells treated with RB51, ABOR+Chitin, ABOR protein, Chitin, and PBS groups on days 0 (before treatment), and the calculated PI [OD of test sample−OD of negative control/OD of negative control].</p>](Images/Articles/60592/f5_small.png)
Figure 5. Comparison of Proliferation Index (PI) in guinea pigs' spleen cells treated with RB51, ABOR+Chitin, ABOR protein, Chitin, and PBS groups on days 0 (before treatment), and the calculated PI [OD of test sample−OD of negative control/OD of negative control].
|
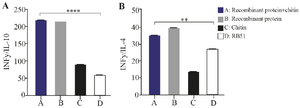
Figure 6. The ratio of the expressed cytokines in different experimental groups. The ratio of IFN-γ to IL-10(I), and IL-4(II) after immunization of guinea pigs.
A: ABOR protein and Chitin. B: ABOR protein. C: Chitin. D: RB51 vaccine.
|
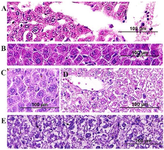
Figure 7. Histopathological evaluation of hepatic tissues belong to groups A to E after injections showed no toxic effects: A) PBS, B) ABOR protein+chitin, C) RB51 Vaccine, D) Chitin, and E) ABOR protein (H& E, Bar= 100 μm).
|
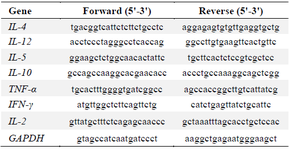
Table 1. The primers used in cytokine genes expression level assay by Real-Time RT-PCR
IL: Interleukin, TNF: Tumor Necrosis Factor, IFN: Interferon.
|
|