The Potential of Human Wharton’s Jelly Mesenchymal Stem Cells Secretome Based Topical Gel for Therapeutic Application
-
 Widowati, Wahyu
Faculty of Medicine, Maranatha Christian University, Bandung, 40164, Indonesia, Tel: +62 819 10040010; E-mail: wahyu_w60@yahoo.com, wahyu.widowati@maranatha.edu
Widowati, Wahyu
Faculty of Medicine, Maranatha Christian University, Bandung, 40164, Indonesia, Tel: +62 819 10040010; E-mail: wahyu_w60@yahoo.com, wahyu.widowati@maranatha.edu
-
Faried, Ahmad
-
Department of Neurosurgery, Oncology & Stem Cell Working Group, Faculty of Medicine, Universitas Padjadjaran, Bandung 40161, Indonesia, Bandung 40161, Indonesia
-
Dr. Hasan Sadikin Hospital, Bandung 40163, Indonesia
-
Rahardja, Fanny
-
Faculty of Medicine, Maranatha Christian University, Bandung 40163, Indonesia
-
Haifa Zahiroh, Fadhilah
-
Biomolecular and Biomedical Research Center Bandung, Aretha Medika Utama, Bandung 40163, Indonesia
-
Firdaus Sutendi , Annisa
-
Biomolecular and Biomedical Research Center Bandung, Aretha Medika Utama, Bandung 40163, Indonesia
-
Azis, Rizal
-
Biomedical Engineering Department of Electrical Engineering, Faculty of Engineering, University of Indonesia, Depok, Indonesia
-
Department of Translational Medical Science, Division of Cancer and Stem Cell, Biodiscovery Institute 3, The University of Nottingham, University Park, United Kingdom NG72RD, Nottingham, United Kingdom NG72RD
-
Kristianlie Ekajaya , Renandy
-
Biology Study Program, Faculty of Mathematics and Science Education, Universitas Pendidikan Indonesia, Bandung 40163, Indonesia
Abstract: Background: Diabetic Foot Ulcer (DFU) might be worsened by neuropathy and vascular issues. This condition can cause 14.3% fatality, stressing the need for effective wound healing therapy. Wound healing is a complex biological process, and human Wharton's Jelly Mesenchymal Stem Cells (hWJMSCs) may help manage DFU treatment issues. This research focuses on utilizing a gel carrier to deliver bioactive substances from Wharton's Jelly Mesenchymal Stem Cells secretome (hWJ-MSCs-Sec) as a possible treatment for DFU.
Methods: To maintain quality, hWJMSCs-Sec is thoroughly mixed with carbomer gel and freeze-dried. ELISA test is performed to determine the characterization of the gel of hWJMSCs-Sec such as Keratinocyte Growth Factor (KGF), Platelet-Derived Growth Factor (PDGF), Hepatocyte Growth Factor (HGF), Epidermal Growth Factor (EGF), and Heparin-Binding EGF-Like Growth Factor (HB-EGF). The antioxidant activity was also measured with Hydrogen peroxide (H2O2), Nitric oxide (NO), and Ferric Reducing Antioxidant Power (FRAP) assay. Proliferation assay was utilized using WST-8 and the wound healing potential was assessed via the migration cell ability of scratched-human skin fibroblast (BJ cells).
Results: The freeze-dried hWJ-MSCs-Sec showed higher levels of KGF, HGF, PDGF, EGF, HB-EGF, and the antioxidant activities compared to fresh hWJ-MSCs-Sec. Additionally, the gel of freeze-dried hWJ-MSCs-Sec exhibited higher levels compared to the gel of fresh hWJMSCs-Sec. This was evidenced by faster closure of scratched wounds on BJ cells treated with hWJMSCs-Sec and freeze-dried hWJ-MSCs-Sec gel.
Conclusion: The freeze-dried hWJ-MSCs-Sec gel exhibits superior quality compared to the non-freeze-dried hWJ-MSCs-Sec gel. This demonstrates that the freeze-drying procedure can maintain the bioactive chemicals found in hWJMSCs-Sec, potentially enhancing the efficacy of this gel in promoting cell regeneration for wound healing.
Introduction :
Diabetic Foot Ulcer (DFU) is an open sore or wound that is caused by complications of diabetes mellitus. DFU affects the feet of diabetes patients and is characterized by sensory, motor, and autonomic neuropathy, as well as macrovascular and microvascular disturbances. DFU can lead to ulcers, infections, gangrene, amputations, and even death. Amputation, a serious outcome of DFU, results in 14.3% of individuals passing away within a year after the procedure 1. Effective strategies for healing DFU wounds must be established to avoid the amputation 2. Efficient wound healing necessitates the coordination of several cellular processes facilitated by multiple growth factors, cytokines, and chemokines. There are four distinct phases involved in the process of wound healing, namely hemostasis, inflammation, proliferation, and remodeling 3. The inflammatory response is triggered to help the body and initiate the healing process by widening the blood vessels and releasing certain signaling molecules such as nitric oxide, bradykinin, histamine, and prostaglandins 4. But in cases where subsequent damage occurs, the inflammation doesn’t resolve as expected, instead, it persists and intensifies, characterized by an abundance of neutrophils releasing Reactive Oxygen Species (ROS) 5. The excessive generation of ROS has been linked to the onset of numerous persistent and degenerative disorders 6. The antioxidant agents are needed to mitigate the harmful effect of ROS and enhance the body’s ability to withstand diverse environmental stress conditions 7.
The process of wound repair involves various growth factors, including Epidermal Growth Factor (EGF), which promotes cell proliferation and cytoprotection 8. Other growth factors such Platelet-Derived Growth Factor (PDGF), Heparin Binding EGF Like Growth Factor (HB-EGF), and Keratinocyte Growth Factor/Fibroblast Growth Factor-7 (KGF/FGF-7) play a significant role in angiogenesis, inflammation regulation, collagen deposition 9, keratinocyte migration, keratinocyte proliferation 10 and reepithelization in wound healing 11. Currently, ongoing research is being conducted in the field of wound therapy with the aim of identifying appropriate treatment agents that can facilitate rapid wound healing while minimizing adverse effects. This pertains specifically to agents that possess elevated concentrations of antioxidants and growth factor proteins. One potential solution that could effectively tackle this matter is the implementation of stem cell treatment.
In the study by Park et al 12, it was discovered that various growth factors, including EGF, PDGF, bFGF, HB-EGF, FGF7/KGF, and HGF, are actively produced within stem cells and released through the secretome. The significant presence of these growth factors suggests their potential in wound healing, particularly in the case of DFU. Wharton's Jelly-derived MSCs (WJMSCs) are a type of stem cell derived from the umbilical cord. WJMSCs have been found to enhance the proliferation and migration of fibroblasts, speed up re-epithelialization, and facilitate wound repair through paracrine signaling 13. The MSCs secretome, which consists of various bioactive substances released into the surrounding cellular environment, exerts a strong impact on nearby tissues 14. The Wharton's Jelly Mesenchymal Stem Cells Secretome (hWJ-MSCs-Sec) has been commonly used to enhance wound healing after severe injuries 15. Therefore, the hWJ-MSCs-Sec can be developed into a gel product for wound healing or DFU treatment. Gel products create a moist environment at the wound site, fostering tissue renewal through granulation and re-epithelialization 16. The excellent adhesive and spreading properties of the gel enhance its application and therapeutic effectiveness, ensuring robust adherence to the target area, uniform spreading. and ultimately optimizing the therapeutic or cosmetic effects 17. To safeguard the active compounds within the secretome, the freeze-drying process is employed. This technique ensures the preservation of proteins within the secretome, enhancing the reliability and consistency of molecular assessments 18.
This study aims to explore the efficacy of topical gel containing hWJMSCs-Sec for the treatment of DFU. This will be achieved through an examination of the gel’s antioxidant properties by its scavenging activity towards Hydrogen peroxide (H2O2), Nitric Oxide (NO), and Ferric Reducing Antioxidant Power (FRAP). The analysis of the proteins content of freeze dried hWJ-MSCs-Sec gel involved the implementation of the Enzyme-Linked Immunosorbent Assay (ELISA) method, focusing on the evaluation of Keratinocyte Growth Factor (KGF), Platelet-Derived Growth Factor (PDGF), Hepatocyte Growth Factor (HGF), EGF, and hHB-EGF. Finally, the in vitro potential of hWJMSCs-Sec gel both freeze dried and fresh for wound healing was assessed by evaluating the cells proliferation and the cells migration of scratched-human skin fibroblast cells (BJ cells).
Materials and Methods :
Formulation of carbomer gel: The carbomer gel formulation was conducted based on the study of Zhang et al 19, with certain alterations. Disperse 7.5 g carbomer 940 slowly into 250 ml aquadest with a continuous stirring using a Stirring Hotplates (Fisher Scientific, 1110217SH). After the carbomer swells in distilled water, add 7.5 ml triethanolamine, 5 ml propylene glycol, and 5 ml glycerin drop by drop while stirring gently until the mixture forms a homogeneous gel. Lastly, add distilled water gently until it reaches 500 ml.
Production of hWJ-MSCs Freeze-Dried hWJ-MSCs-Sec Gel (FDSG): Preparation of hWJMSCs Secretome: The hWJMSCs passage 5 were obtained from Aretha Medika Utama Bandung, Indonesia. The cultivation protocol for Human Wharton’s Jelly Stem Cells (hWJ-MSCs) was elucidated by Widowati, et al 20 and has been characterized using multipotent differentiation experiments and surface phenotypic analysis in prior studies 21. Cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) High Glucose (Biowest, L0475-500) with 10% FBS, 1% Antibiotic and Antimycotic (ABAM) (Biowest, L0010-100), 0.1% Gentamicin (Gibco, 15750060), 1% Amphotericin B (Biowest, L0009-100), and 1% Nanomycopulitin (Biowest, L-X16-100) and incubated with CO2 Incubator (Thermo IH3543) at 37°C with 5% CO2. After the hWJMSCs reached 80-90% confluence, the culture medium was collected and centrifuged with Refrigerated Centrifuge (MWP 260r) at 3000×g for 4 min at 37°C. The resulting supernatant, containing secreted factors, was purified using a Durapore (Millipore Corporation, SLGV 033 RS) filter unit 22.
Production of Freeze-Dried hWJ-MSCs-Sec gel: The preparation of the gel involves incorporating fresh and fresh hWJMSCs-Sec into the carbomer gel in three sequential stages, following a specific formula (P1: 6 g carbomer gel+3 ml hWJ-MSCs-Sec, P2: 6 g carbomer gel+4.5 ml hWJ-MSCs-Sec, P3: 6 g carbomer gel+6 ml hWJ-MSCs-Sec), each mixture is stirred gently until it reaches a homogeneous consistency 23. Subsequently, the hWJ-MSCs-Sec gel is placed in a small tray and placed inside the vacuum freeze-drying machine used for the lyophilization method with a freeze dryer (FD-F-CE, China) tool 24. The lyophilization process using a freeze dryer was carried out for 42 hr at a temperature of 35-50°C.
Proteins assay: The Human KGF (E-EL-H0092), HGF (E-EL-H0084), PDGF (E-EL-H2211), EGF (E-EL-H0059), and HB-EGF (E-EL-H2667) level were assayed using the Elabscience kit, based on manufacturing protocols. The ELISA Assay quantification was done to measure the hWJ-MSCs-Sec gel characteristics and the protein level of hWJ-MSCs-Sec gel 25.
Antioxidant assay: H2O2 Scavenging Activity Assay: hWJ-MSCs-Sec gel was added 60 μl to the 96-well microplate. In the control and blank wells, 12 μl of 1 mM ferrous ammonium sulfate (Sigma Aldrich, 215406) were introduced. Following this, dimethyl sulfoxide (DMSO) (Supelco, 1.02952.1000) was added to the control (63 μl) and blank (90 μl) wells. Then, 3 μl of 5 mM H2O2 (Merck, 1.08597.1000) was added to each well of the microplate. After incubating time in a dark room for 5 min, 1,10-phenanthroline (Sigma Aldrich, 131377) was added (75 μl) into each well. Following another 10-minutes incubation, absorbance at 510 nm was measured using a spectrophotometer 26. H2O2 scavenging activity was determined using the formula:
% scavenging activity= Control absorbance-sample absorbancecontrol absorbance ×100
NO Scavenging activity assay: Sodium nitroprusside 10 mM (Merck, 106541) in PBS (Gibco, 1740576) as much as 40 μl was mixed with 10 μl of the hWJ-MSCs-Sec gel sample. After incubating the mixture at room temperature for 2 hr, 100 μl of Griess reagent containing 1% sulfanilamide (Merck, 111799), 2% H3PO4 (Merck, 100573), and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride (Sigma Aldrich, 222488) was added to each well of 96-well plate and the absorbance was measured at 546 nm 27. NO scavenging activity was determined using the formula:
% scavenging activity= Control absorbance-sample absorbancecontrol absorbance ×100
FRAP assay: To start preparing the reagent, 10 ml of 300 mM acetate buffer (pH=3.6) was combined with 1 ml of 20 mM ferric chloride hexahydrate (Merck 1.03943.0250) and 1 ml of 10 mM 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) (Sigma- Aldrich, T1253). Following that, 7.5 μL of hWJ-MSCs-Sec gel sample and 142.5 μl of the FRAP reagent were introduced into the wells and incubated at 37°C for 6 min before it got measured at 593 nm 28. FRAP scavenging activity was determined using the formula:
% scavenging activity= Control absorbance-sample absorbancecontrol absorbance ×100
Proliferation assay: The human skin fibroblast cell line (BJ cells) (ATCC, CRL-2522) was cultured based on previous study 29. As much as 5×103 cells were grown in a 96 well plate. After reaching confluence, the cells were treated with various samples, while untreated cells act as a negative control (NC). WST-8 were introduced after 24 hr of treatment and the absorbance was read at 595 nm 30.
Cells migration: To determine the ability of hWJ-MSCs-Sec gel in wound healing, cells migration assays were conducted on scratched-human skin fibroblast cells as wounded cells model. The fibroblast cells were plated on a 24 well plate at a density of 7×103. After reaching 90% confluence, the cells were scratched across the well to create a wound model by using a blue tip. Cells were then treated with the hWJ-MSCs-Sec gel samples, and cell without treatment act as a NC. After 24 hr, the scratched area was measured under the microscope using Image J software 31.
Statistical analysis: The resulting data represents the mean ± standard deviation from tree replicate measurements of each sample. Comparisons of treatments were undertaken using ANOVA, with a significance level of p<0.05. The post hoc test, namely Tukey Honestly Significant Difference, along with the Dunnett T3 test with a 95% confidence interval, was utilized. The statistical analysis was conducted using SPSS software (version 20.0), and the findings were visualized using GraphPad Prism program (version 9.0).
Results :
Protein content of Freeze-Dried hWJMSCs-Sec gel: The influence of hWJMSCs-Sec treatments on the levels of Growth Factor Proteins are elucidated in figure 1. As shown in figure 1, the hWJMSCs-Sec treatment in the FDSG regimen demonstrated an increasing trend in protein levels alongside the addition of hWJMSCs-Sec volume to the carbomer gel. On the other hand, the Freeze-Dried hWJ-MSCs-Sec (FDS) treatment significantly increased the levels of KGF, HGF, PDGF, EGF, and HB-EGF compared to other treatments, especially fresh hWJ-MSCs-Sec (S). Additionally, Freeze-Dried Gel Based (FDGB) and Freeze-Dried Medium Basal (FDMB) were used as negative control groups (p<0.05).
Antioxidant activity of Freeze-Dried hWJMSCs-Sec gel: To determine its potential as a wound healing treatment, antioxidant testing was conducted by evaluating its ability to scavenge H2O2, NO, and FRAP (Figures 2-4). The antioxidant activity of FDSG exhibits a significant superiority in all antioxidant tests compared to the Secretome (S) and hWJMSCs-Sec Gel (SG). The results of antioxidant activity tend to change with different hWJMSCs-Sec Gel formulation. FDSG itself exhibits the highest antioxidant activity in the formulation of most concentrated hWJMSCs-Sec Gel, with the highest concentration being 400 µg/ml.
Proliferation effect of Freeze-Dried hWJMSCs-Sec gel: Proliferation assays were conducted to assess the cells viability of freeze-dried hWJMSCs-Sec and freeze-dried hWJMSCs-Sec gel on human skin fibroblast cells. The results of these assays are presented in figure 5. The addition of hWJMSCs-Sec at various concentrations did not exhibit any toxic activity towards fibroblast cells. Conversely, cells treated with hWJMSCs-Sec demonstrated enhanced proliferation compared to the Negative Control (NC). The freeze-dried secretome showed superior cell viability compared to the S samples. These findings indicate that the freeze-drying process preserves important bioactive components in hWJMSCs-Sec and is non-toxic to fibroblast cells.
Migration of fibroblast cells after the treatment with Freeze-Dried hWJ-MSCs-Sec gel: The proliferation and migration of fibroblast are crucial for the process of skin repair/regeneration. Therefore, migration assays were conducted on fibroblast cells that were subjected to scratching to assess the wound healing process by evaluating the effects of hWJMSCs-Sec gel on cell proliferation and migration abilities. The migration data are shown in figure 6. The results demonstrate increased cell migration values with higher addition of hWJMSCs-Sec. FDS exhibits the highest efficacy in promoting cell migration, surpassing even that of S itself. Similarly, FDSG demonstrates superior efficacy compared to S, closely resembling the outcome of FDS. This substantiates that the growth factors within the secretome are preserved through the freeze-drying process.
Discussion :
Wharton's Jelly is widely regarded as a highly reliable and abundant source of mesenchymal stem cells. In the preceding investigation, a comprehensive analysis of hWJMSCs was undertaken. It has been demonstrated that hWJMSCs exhibit a high expression of CD90, CD105, CD44, and CD73, while displaying a negative expression of CD11b, CD19, CD34, CD45, and HLA-DR 21. In this experiment, passage 5 of the hWJMSCs cells was selected due to its notable and consistent proliferation 32. Carbomer is utilized as the primary component to formulate the secretome from WJMSCs into a gel form. Freeze-drying, also known as lyophilization, is a crucial technique utilized in this study to safeguard the integrity of hWJMSCs-Sec, particularly due to their susceptibility to heat-induced damage, as emphasized by previous research findings 33. This method involves the removal of moisture under low temperatures and reduced pressure, mitigating the potential harm caused by heat to the sensitive cellular structure of hWJMSCs-Sec. Moreover, freeze-drying serves as a promising approach for the preservation of biomaterial and natural cells in a desiccated state, a process that enhances their stability during storage and transportation. The significance of this technique lies in its ability to prolong the shelf life of hWJMSCs-Sec by maintaining their structural and functional characteristics, even at room temperature. Recent studies, such as the work by Merivaara et al 34, underscore the efficacy of freeze-drying in providing optimal storage conditions, making it a valuable method for the long-term preservation of biological materials.
ELISA results have illuminated a significant disparity between freeze-dried hWJ-MSCs-Sec and its conventional counterpart, particularly concerning the protein content of critical growth factors. This disparity is marked by notably higher concentrations of several growth factors, specifically KGF, HGF, PDGF, EGF, and HB-EGF, within the freeze-dried hWJ-MSCs-Sec. These findings bear profound implications for the applications of hWJ-MSCs-Sec in regenerative medicine and tissue engineering. The elevated content in KGF, HGF, PDGF, EGF, and HB-EGF in freeze-dried hWJ-MSCs-Sec underscores its potential in accelerating wound healing and tissue repair, demonstrating a promising avenue for therapeutic interventions 35.
Growth factors are signaling molecules that play a critical role in cell growth, differentiation, and survival 36. The five growth factors discussed in this article are all involved in different aspects of wound healing, from inflammation and angiogenesis to cell proliferation and extracellular matrix deposition. KGF is a growth factor that is primarily produced by keratinocytes, the cells that make up the epidermis 37. KGF is a potent stimulant of keratinocyte proliferation and differentiation. While HGF is a potent stimulant of cell proliferation, migration, and differentiation. Together, they play a role in angiogenesis and inflammation 38,39. The present investigation observed a positive correlation between the formulation of hWJ-MSC-Sec and the levels of KGF and HGF in FDSG. Previous research has elucidated that the levels of HGF exhibit an elevation in rats afflicted with DFU following treatment with secretome derived from umbilical cord mesenchymal stem cells (UCMSCs) 40.
PDGF is an additional growth factor that can be specifically targeted for the therapy of DFU. So far, the Food and Drug Administration (FDA) has granted permission to PDGF for its capacity to expedite the process of wound healing 41 because of its capacity for attracting fibroblast 42. PDGF was detected in FDSG samples as well in this analysis. This finding suggests that FDSG exhibits promise in the treatment of DFU. EGF and HB-EGF was also found in FDSG, as a growth factor that is produced by a variety of cells, including keratinocytes, fibroblasts, and macrophages 43. Kuo et al 44 observed that the incorporation of stem cells resulted in a significant increase in the concentration of EGF on the accelerated healing of wounds in individuals with diabetes. Another study stated that the addition of HB-EGF resulted in an increase in the formation of granulation tissue in mice with diabetic wounds 45. In all protein tests performed, FDS exhibited the highest efficacy among the samples, including FDSG. This could be influenced by the addition of carbomer gel, resulting in a lower concentration of active ingredients in the secretome gel compared to the secretome itself. As seen in figure 1, freeze-dried carbomer gel (FDGB) did not show any presence of growth factor proteins.
In addition to growth factors, the presence of oxidative stress within the wound will also exert an influence on the process of wound healing. High levels of H2O2 at a wound site serve as a signal for initiating chemotactic and inflammatory response 46. The inflammatory response can also be exacerbated by the presence of NO. Nitric oxide plays a pivotal role in regulating three crucial aspects of wound healing: vascular homeostasis, inflammation, and antimicrobial activity 47. These radical compounds can be identified as potential targets for wound healing interventions. The gel hWJ-MSCs-Sec exhibits a high antioxidant level (Figures 2-4). After the freeze-drying process, FDSG still exhibited remarkably high antioxidant results. This data provides evidence that the freeze-drying process effectively preserves the active compounds within the hWJ-MSCs-Sec gel. Based on the results, it was proven that FDSG comprises growth factor protein and high antioxidant levels, rendering it a promising DFU agent.
To further explore the potential of gel secretome as a wound healer, cell proliferation and migration assays on human skin fibroblast cells were conducted. FDSG shows no toxic effect towards the fibroblast cells. Instead, the cell’s viability increased after the treatment with FDSG, FDS, and S itself (Figure 5). Cell migration is pivotal in developmental processes within cells. MSCs exhibits multipotent characteristic and reparative abilities, rendering them promising sources of stem cells for degenerative therapy. MSCs possess various chemical factors such as chemokines, cytokines, and other growth factors, which support MSC migration to wound sites 48. Growth factors like EGF, FGF, TGFs, VEGF, and PDGF play a crucial role in regulating cell division, differentiation, and remodeling, 49 which facilitate cell proliferation and cell migration 50. The proposed mechanism of hWJMSCs-Sec as a wound healing agent was summarized in figure 7. The study results indicate that hWJMSCs-Sec contains several growth factors, thus supporting fibroblast cells to close faster after treatment with hWJMSCs-Sec. FDS-100 µg/ml and FDSG-100 µg/ml exhibited superior abilities with wound closure of 95.62% and 90.40%, when compared to NC. This result also further proves that the freeze-drying process on hWJMSCs-Sec gel can maintain quality by dehydrating the product into powder form. The dehydrated product also supports longer storages with good quality 51. However, this study was hindered by a lack of verification assays. Further in vitro as well as in vivo approaches are needed to support this study.
Conclusion :
In this study, hWJ-MSCs-Sec is thoroughly mixed with the carbomer gel and freeze-dried to maintain quality stability. This is evidenced by the fact that after undergoing the freeze-drying process, the protein levels of growth factors and antioxidant activity from hWJ-MSCs-Sec still yielded excellent results, even surpassing those that did not undergo the freeze-drying process. The application of freeze-dried hWJ-MSCs-Sec via carbomer gel has demonstrated the preservation of growth factors and antioxidant properties. This endows it with exceptional migratory capabilities towards the wound site, indicating potential for wound healing therapy.
Ethical approval :
This research obtained ethical approval from the Research Ethics Committee of the Faculty of Medicine at Maranatha Christian University (Approval No: 097/KEP/VII/2020, dated July 25th, 2020).
Acknowledgement :
The authors express their gratitude Maranatha Christian University, Bandung, West Java, Indonesia, for grant of Research for Productive Lecturer (No: 011/SK/ADD/UKM/IV/2024), and to Minister of Education, Culture, Research and Technology of the Republic of Indonesia (Applied Research 2024 with grant number 106/E5/PG.02.00.PL/2024 and 007/SP2H/RT-MONO/LL4/2024) and the Center for Biomolecular and Biomedical Research, Aretha Medika Utama, Bandung, West Java, Indonesia, for providing laboratory facilities and methodology. Special thanks to Hanna Sari Widya Kusuma, Nindia Salsabila Mia Dewi, Adilah Hafizha Nur Sabrina, and Vini Ayuni for their valuable assistance.
Funding: The research is funded by Maranatha Christian University, Bandung, West Java, Indonesia for grant of Research for Productive Lecturer (No: 011/SK/ADD/UKM/IV/2024) and the Minister of Education, Culture, Research, and Technology of the Republic of Indonesia under the Applied Research 2024 with grant number 106/E5/PG.02.00.PL/2024 and 007/SP2H/RT-MONO/LL4/2024.

Figure 1. Effect of various treatments on hWJMSCs-Sec toward growth factor proteins level.
- A) KGF, B) HGF, C) HB-EGF, D) PDGF, E) EGF. The data were presented as mean ± standard deviation. Different superscript letters on Figure A (a, b, c, cd, d, e, f), and B, C, D, E (a, b, c, d, e, f, g) showed significant differences among treatments at p<0.05 (Data was analyzed using ANOVA followed by Tukey HSD post hoc test). Samples included hWJMSCs-Sec (S), Freeze-dried hWJMSCs-Sec (FDS), Freeze-dried Medium Basal (FDMB), Freeze-Dried hWJMSCs-Sec Gel (FDSG), and Freeze-dried Gel Based (FDGB). Number on samples indicate the ratio of gel to hWJMSCs-Sec, 1) 6 g: 3 ml, 2) 6 g: 4.5 ml, and 3) 6 g: 6 ml.
|
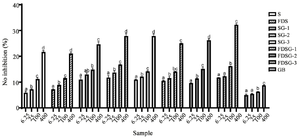
Figure 2. Antioxidant level of hWJ-MSCs-Sec gels in various formulations by its ability to scavenge H2O2.
*The data were presented as mean ± standard deviation. Different letters (a, ab, b, bc, c, d) showed significant variation among concentration at p<0.05. Data was analyzed using ANOVA and followed by Tukey HSD & Dunnett T3 post hoc test. Samples included hWJ-MSCs-Sec (S), Freeze-dried hWJ-MSCs-Sec (FSD), hWJ-MSCs-Sec Gel (SG), Freeze dried hWJ-MSCs-Sec gel (FDSG), and Carbomer gel base (GB). Number on samples indicate the ratio of gel to hWJMSCs-Sec, 1) 6 g: 3 ml, 2) 6 g: 4.5 ml, and 3) 6 g: 6 ml.
|
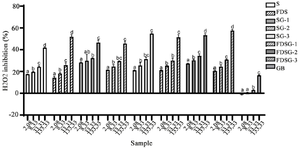
Figure 3. Antioxidant level of hWJMSCs-Sec gels in various formulations by its ability to scavenge NO.
*The data were presented as mean ± standard deviation. Different letters (a, ab, b, bc, c, d) showed significant variation among concentration at p<0.05. Data was analyzed using ANOVA and followed by Tukey HSD & Dunnett T3 post hoc test. Samples included hWJ-MSCs-Sec (S), Freeze-dried hWJ-MSCs-Sec (FDS), hWJ-MSCs-Sec Gel (SG), Freeze dried hWJ-MSCs-Sec gel (FDSG), and Carbomer gel base (GB). Number on samples indicate the ratio of gel to hWJMSCs-Sec, 1) 6 g: 3 ml, 2) 6 g: 4.5 ml, and 3) 6 g: 6 ml.
|
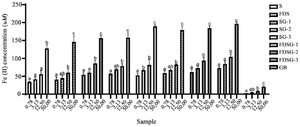
Figure 4. Antioxidant level of hWJ-MSCs-Sec gels in various formulations by its ability to scavenge FRAP.
*The data were presented as mean ± standard deviation. Different letters (a, ab, b, c) showed significant variaton among concentration at p<0.05. Data was analyzed using ANOVA and followed by Tukey HSD & Dunnett T3 post hoc test. Samples included hWJ-MSCs-Sec (S), Freeze-dried hWJ-MSCs-Sec (FDS), hWJ-MSCs-Sec Gel (SG), Freeze dried hWJ-MSCs-Sec gel (FDSG), and Carbomer gel base (GB). Number on samples indicate the ratio of gel to hWJMSCs-Sec, 1) 6 g: 3 ml, 2) 6 g: 4.5 ml, and 3) 6 g: 6 ml.
|
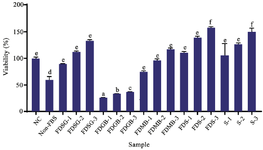
Figure 5. Proliferation effect of hWJMSCs-Sec gel towards human fibroblast cells.
*The data were presented as mean ± standard deviation. Different letters (a, b, c, d, e, f) showed significant variation among concentration at P<0.05. Data was analyzed using T-Test. Samples included: Negative Control: untreated-cells (NC), Cells without FBS (Non-FBS), Cells+hWJMSCs-Sec (S), Cells+Freeze-dried hWJMSCs-Sec (FDS), Cells+Freeze-dried Medium Basal (FDMB), Cells+ Freeze-Dried hWJMSCs-Sec Gel (FDSG), and Cells + Freeze-dried Gel Based (FDGB). Number on samples indicates the concentration used for treatment, 1) 1.3 µg/ml, 2) 12.5 µg/ml, and 3) 100 µg/ml. While for Secretome (S), the number indicates the concentration of 1) 3.25%, 2) 12.5%, and 3) 50%.
|

Figure 6. Effect of various treatments on hWJMSCs-Sec toward the migration of human skin fibroblast cells.
*The data were presented as mean±standard deviation. Different letters (a, b, c, d, e, f, g, h) showed significant variation among samples at p<0.05. Data was analyzed using T-Test. Samples included: Scratched-cells (NC), Scratched-cells without FBS (Non-FBS), Scratched-cells+hWJMSCs-Sec (S), Scratched-cells+Freeze-dried hWJMSCs-Sec (FDS), Scratched-cells + Freeze-dried Medium Basal (FDMB), Scratched-cells+Freeze-Dried hWJMSCs-Sec Gel (FDSG), and Scratched-cells + Freeze-dried Gel Based (FDGB). Number on samples indicate the concentration used for treatment, 1) 1.3 µg/ml, 2) 12.5 µg/ml, and 3) 100 µg/ml. While for Secretome (S), the number indicates the concentration of 1) 3.25%, 2) 12.5%, and 3) 50%.
|
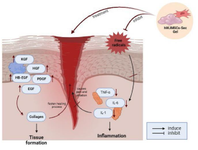
Figure 7. Proposed mechanism of hWJMSCs-Sec gel as a wound healing agent.
* The treatment of hWJMSCs-Sec gel into the wounded skin can regulate some growth factors such as KGF, HGF, HB-EGF, PDGF, and EGF. The upregulation of these growth factors can induce the production of collagen, which plays a crucial role to regenerate tissue formation. The high antioxidant hWJMSCs-Sec gel also has the ability to scavenge free radicals, and inhibit the excessive inflammation that can cause irritation, redness, and pain in the wound.
|
|