Bactericidal Activity of Serum by Brucella Abortus RB51 Outer Membrane Protein’s Combined by Brucella Abortus S99 Lipopolysaccharide Induction
-
Hajizadeh Sisakht, Behnam
-
Department of Bacteriology, Faculty of Veterinary, Islamic Azad University, Science & Research Campus, Tehran, Iran
-
Khaledi, Mansoor
-
Department of Microbiology and Immunology, School of Medicine, Shahrekord University of Medical Sciences, Shahrekord, Iran
-
Afkhami, Hamed
-
Department of Microbiology, Faculty of Medicine, Shahed University, Tehran, Iran
-
Rouhi, Saber
-
Resident of Large Animal Internal Medicine, Department of Clinical Sciences, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
-
Sepehrnia, Saeed
-
Department of Immunology, Faculty of Medicine, Shahed University, Tehran, Iran
-
Fanaee, Vahideh
-
Department of Microbiology and Immunology, School of Medicine, Shahrekord University of Medical Sciences, Shahrekord, Iran
-
Karimi, Hannaneh
-
Department of Microbiology, Rasht Islamic Azad University, Rasht, Iran
-
 Malekzadegan, Yalda
Department of Microbiology, Saveh University of Medical Sciences, Saveh, Iran, Tel: +98 86 48503151; Fax: +98 86 42249547; E-mail: Malekzadeganyalda@gmail.com
Malekzadegan, Yalda
Department of Microbiology, Saveh University of Medical Sciences, Saveh, Iran, Tel: +98 86 48503151; Fax: +98 86 42249547; E-mail: Malekzadeganyalda@gmail.com
-
 Fathi, Javad
Department of Bacteriology and Virology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran , Tel: +98 86 48503151; Fax: +98 86 42249547; E-mail: javadfathi70@yahoo.com
Fathi, Javad
Department of Bacteriology and Virology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran , Tel: +98 86 48503151; Fax: +98 86 42249547; E-mail: javadfathi70@yahoo.com
-
 S. Sadati , Mahdi
Department of Microbiology, Faculty of Biological Sciences, Islamic Azad University Tehran-North Branch, Tehran, Iran , Tel: +98 86 48503151; Fax: +98 86 42249547; E-mail: university.ac55@gmail.com
S. Sadati , Mahdi
Department of Microbiology, Faculty of Biological Sciences, Islamic Azad University Tehran-North Branch, Tehran, Iran , Tel: +98 86 48503151; Fax: +98 86 42249547; E-mail: university.ac55@gmail.com
Abstract: Background: Brucellosis vaccines are designed to induce cellular immunity. An effective brucellosis vaccine could induce both cellular and humoral immunity. Serum Bactericidal Assay (SBA) is an important method for determining vaccine humoral immunity. This study is the first to observe humoral immunity in brucellosis by SBA.
Methods: Extracted Brucella abortus (B. abortus) Lipopolysaccharide (LPS) and Outer Membrane Proteins (OMPs) were injected into rabbits. Group 1 was injected with 25 µg of LPS, Group 2 was injected with 50 µg of OMPs, and Group 3 was injected with 1 ml of combined vaccine, 3 times every 2 weeks. The groups were challenged with B. abortus 544 in the second injection. Sera were separated 2 weeks after the last injection. SBA was performed, and each well was streak-cultured into a plate of Brucella agar. A colony count was done for each plate.
Results: Results have shown, the third injection of the combined vaccine had the highest titer of 1/64, and the efficacy of the vaccine was 87.71%.
Conclusion: As a conclusion, the results of this study showed that LPS and OMP's from B. abortus can provide acceptable immunity.
Introduction :
The first effective vaccine against brucellosis was the live attenuated vaccine Brucella abortus (B. abortus) S19 strain. Although this vaccine protects cattle against B. abortus, its side effects have led it to be used cautiously 1,2. Despite these complications, the Brucella melitensis (B. melitensis) vaccine strain, Rev-1 leads to the control of brucellosis in sheep and goats, only in endemic areas 3. Although previous studies have examined the live attenuated B. abortus 45/20 vaccine, this strain may still cause disease in some cases. B. abortus strains RB51 vaccine can protect cattle against B. abortus with less side effects 4. The vaccine is a preventive tool used to eradicate brucellosis.
Classical serological tests detect antibodies against the Lipopolysaccharide (LPS) antigens induced by vaccination with S19 or Rev. 1 or exposure to virulent field strains 5. Therefore, no specific serological tests can distinguish between animals vaccinated with S19 and Rev-1 or a natural Brucella strain infection to detect the disease. Recently, mutant strains of B. abortus RB51 were proposed to be used in the immunization of cattle. Vaccines used for brucellosis are ∼70% efficacious and may cause disease in some vaccinated animals 6.
The major Outer Membrane Proteins (OMPs) of Brucella spp. were first introduced in the 1980s 7. They were soon characterized as potential immunogenic and protective antigens. They were classified according to their apparent molecular mass as group 1, 36-38 kDa OMPs, group 2 porin proteins and 31-34 and 25-27 kDa OMPs, which belong to the group 3 proteins 7. The use of recombinant protein technology and Monoclonal antibodies (MAbs) has shown that the major OMPs appear to be of little relevance as antigens in smooth (S) B. abortus or B. melitensis infections, i.e. low or no protective activity in the mouse model of infection and low or no immunogenicity during host infection. However, group 3 proteins, in particular Omp31, appear as immunodominant antigens in the course of rough (R) Brucella ovis (B. ovis) infection in rams and as an important protective antigen in the B. ovis mouse model of infection. The major OMP genes display diversity and specific markers have been identified for Brucella species, biovars, and strains, including the recent marine mammal Brucella isolates for which new species names have been proposed.
The B. abortus LPS, compared to the Enterobacteriaceae, has an unusual structure that is significant in making T-cell-mediated responses 8. It can induce IgM and IgG. In addition, according to new studies, the B. abortus LPS can show adjuvanticity 9. Moreover, it is also a Hapten. The LPS has a crucial role in the pathogenesis of Brucella ssp., therefore, by the characteristics described above, it is likely to be considered as a qualified vaccine candidate 10.
The susceptibility of micro-organisms to antimicrobial agents is often measured in vitro by measuring the inhibiting activity of growth. These measurements often do not provide sufficient information to determine the appropriate treatment of certain infections, such as endocarditis 11. In addition, a specific β- Lactam antibiotic, which has bactericidal activity, does not have bactericidal activity for all organisms 12. Therefore, there is a requirement for new experimental methods which can observe the bactericidal activity in optimal conditions. Schlichter and Mac Lean are considered the founders of the Serum Bactericidal Assay (SBA) methods. They discovered the inhibitory effects of blood serum. However, they were unable to define criteria for the characterization of bactericidal effects. Fisher completed Schlichter’s discovery path by culturing the tubes, which showed no visible bacterial growth 13. However, Fisher disclaimed the urge to determine the concentration levels of bactericidal effect 14. Since killed or weakened vaccines may cause pathogenicity, the use of biological and immunological compounds that can induce antibody production and cause the immunity is a solution to prevent the side effects of killed and weakened vaccines.
In this study, we aimed to determine the bactericidal activity of the serum induced by the potential B. abortus LPS and OMP’s combined subunit vaccine.
Materials and Methods :
Strains used in this study: B. abortus S99 was obtained from the Bacterial Cell Bank of Pasteur Institute of Iran (CSBPI) as the strain for smooth LPS extraction. B. abortus RB51 was provided as a live attenuated vaccine, which was used after resuscitation, as the source of the OMP’s extraction. B. abortus 544 was provided by the Department of Bacteriology, Tarbiat Modares University, and used as the challenge strain for vaccine immunization in mice and rabbits.
LPS extraction: The LPS of B. abortus S99 was extracted and purified using a modified hot phenol method based on Westphal's (1986) technique. Phenol at 68°C was added to a batch cultured B. abortus S99 in a fermentor (Novo-Paljas Netherland) and then centrifuged at 2900 rpm. To separate the proteins and nucleic acids, cold methanol was added to the phenolic phase after the separation. Trichloroacetic acid (0.5 g/l of solution) was added and stirred at 4°C for 30 min. Following centrifugation, the supernatant underwent dialysis (cut-off=20 kDa) three times in distilled water over 24 hr. Next, the white precipitate collected inside the dialysis bag was placed in 100 ml vials and lyophilized 15.
LPS Detoxification: The LPS obtained was detoxified by the addition of 0.2 N NaOH to the purified LPS, placed at 100°C for 2 hr. After it cooled down, the pH was set to 7 by 0.1 N HCL. The fatty acids, released by performing dialysis against distilled water for 4 days, were cleared. The dialysate (containing D-LPS) was subsequently lyophilized. Quality assurance tests, including Limulus Amebocyte Lysate (LAL), Bradford test (it is based on the shift in absorbance maximum of Coomassie Brilliant Blue G-250 dye from 465 to 595 nm following binding to denatured proteins in solution) 16, and bioassay [LPS toxicity was compared with its detoxified form using the LAL (Limulus Amoebocyte Lysate) method], were also performed to ensure LPS detoxification based on reported protocols 17.
OMP extraction: Its moisture weight was suspended in 10 mM Tris buffer (Sigma) with ethylenediamine, trichloroacetic acid, and 10 mM EDTA (W/V). The 100% power for 4 min sonication was done within half a minute at 3500 rpm at 4°C and was then centrifuged. Then the cell-free fluid was centrifuged for 60 min at 42000 rpm and ultracentrifugation was done at 4°C. The precipitate was suspended in 20 ml sodium N-Lauryl sarcosinate 2%, added to 0.1 M Tris buffer containing 10 mM EDTA and kept at room temperature for 1 hr. The sample was ultracentrifuged at 42000 rpm for 1 hr at 4°C. The precipitated OPMs in the tube were solubilized in 15 ml of water, then dialyzed against 0.2 M NaCl with three replacements every 8 hr for 24 hr at 4°C. The OMP deposits were then dissolved in 15 ml of distilled water and dialyzed against 0.2 M NaCl. The dialyzed OMPs were divided into sterile 20 and 50 ml glass vials after passing through 0.22 μm filters, and stored at -20°C. Quality control tests were also conducted 18.
Combination of the B. abortus LPS and OMPs: The combined vaccine contains 25 mg/ml LPS and 50 µg/ml OMPs. 10 ml of the vaccine was provided 19.
Colony count of mice spleens: First, the mice were sacrificed and the spleens removed and crushed in plates containing 1 ml saline. The original spleen concentration was diluted 110, 1100 and 11000 and then streak cultured on Brucella agar medium and incubated at 37°C. The plates were controlled for up to 1 week. The colonies formed on the agar plate, were counted next 20.
Immunization of mice and rabbits: One week to 10 days before the challenge, the mice were adapted to the test conditions and then divided into random groups. Five BALB/c mice were each injected with 0.2 ml of the combined vaccine, following the same injection schedule as the rabbits. Four groups of two white New Zealand rabbits each were selected. Group 1 received a 25 µl LPS injection, Group 2 received a 50 µl injection of the OMPs, and the third group received a 1 ml injection of the combined vaccine. The injections were administered twice, with a 14-day gap between each. To induce brucellosis in all groups, they were injected with the challenge strain of B. abortus 544, two weeks after the initial injection. Two weeks after the final injection, blood was drawn from all groups and their sera were separated to conduct a SBA 21.
Serum Bactericidal Assay (SBA): SBA was performed by, serial two-fold dilution of the group’s sera in Dulbecco's Phosphate-Buffered Saline (DPBS); by placing 50 µl DPBS in 12 to 1128 wells (the second well in each row to the last well). The first well of each group of serum was placed with 100 µl of undiluted serum. 50 µl of the first well content were placed in the 12 well and serially diluted by the same sampler tip to the 1128. Then, 50 µl of 105 B. abortus 544 concentrations was placed in each well as a challenge 22.
The following controls were also considered: Negative serum: 50 µl Negative serum+50 µl 105 bacterial concentration (B. abortus 544)+50 µl DPBS, Cellular Control: 50 µl inactivated rabbit serum+50 µl 105 bacterial concentration+50 µl DPBS, Positive Control: 50 µl Antigen (LPS) positive serum+50 µl 105 bacterial concentration+50 µl DPBS, Complement Control: 50 µl active serum+50 µl 105 bacterial concentration+50 µl DPBS, Bacterial Control: only 50 µl 103 bacterial concentration.
After completing the aforementioned steps, the microplate was incubated at 37°C for 30 min. Subsequently, 1 µl of each well was transferred to Brucella agar plates and streak cultured immediately. Following this, all plates were placed in the incubator at 37°C for 72 hr. The colonies present on all plates were then enumerated. Any plate counts equal to or less than 50% of the colonies on the control plates were deemed positive.
Results :
Results for the mice spleen bacterial count: To assess the immunogenicity of the vaccinated groups, B. abortus strain 544 was injected into the peritoneum of mice. Bacterial counts were determined four weeks after the challenge. Figure 1 depicts the bacterial isolation results from the spleens immunized with the combined vaccine group (vaccine+OMP), revealing significantly fewer colonies compared to the group vaccinated with the live vaccine (Figure 2). The immunity induced by the combined vaccine is 93.3%, while the live vaccine yields 73.3%. As shown in Figure 3, the immunogenicity of combined vaccine (OMP+LPS) was 87.71% and the percentage of live vaccine immunogenicity RB51 was 57.14%.
Results for the bactericidal activity of sera: As the serum bactericidal activity evaluation is a crucial vaccine immunological test, we conducted a serum bacterial assay on sera from immunized animals challenged by the B. abortus 544 strain. The results indicated that antibodies against the combined vaccine appeared on day 45, two weeks after the final vaccine injection. The positive bactericidal titer of this group was 164. Stimulation of antibodies against the combined vaccine was significant, compared to all groups at this time. Table 1 shows the overall efficacy of the combined vaccine vs. the RB51 live vaccine.
Discussion :
While the S19 and RB51 live vaccines used against brucellosis are generally safe, there have been instances where they have caused disease in animals or humans, particularly in those involved in the cultivation of bacteria. Furthermore, distinguishing between vaccinated and infected animals through natural means becomes very difficult after inoculation with these vaccines. Consequently, there has been a growing focus on developing a subunit vaccine for brucellosis, as the use of conventional vaccines in humans poses significant challenges. As a result, the development of a brucellosis subunit vaccine has become a critical area of research.
A variety of protein and non-protein antigens of Brucella, or a combination of them, have been proposed as vaccine candidates. Induction of immunity against brucellosis requires both immune responses, particularly cellular immunity. The characteristics of pure Brucella antigen are not sufficient to induce these responses. Therefore, an effective subunit vaccine against brucellosis seems likely to be a combination of different Brucella antigens 19,23,24.
Generally, pure LPS injection can induce some antibodies, but it does not provide strong immune system stimulation. This is because LPS functions as a thymus-independent antigen. The LPS was derived from B. abortus S99, a smooth strain used at the Pasteur Institute of Iran to produce antigens. This component of the vaccine was considered the hapten due to its immune system stimulation. To address the vaccine's shortcomings, the OMP's of B. abortus RB51 were utilized, as the candidates do not provide long-lasting immunity.
Efforts to develop brucellosis vaccines have been made worldwide. Despite these efforts, no effective vaccine with minimal side effects has been developed for use in humans and animals. In order to develop an effective vaccine against brucellosis, now Smooth Brucella strains: B. abortus S19, B. melitensis Rev-1 and Rough Brucella strains: B. abortus RB51 are used in many countries 23,25,26.
While S19 and Rev-1 have drawbacks such as inducing abortions in pregnant animals and being pathogenic in humans, the more significant issues lie in the production of specific antibodies against O-LPS, and the cross-immunity observed in conventional serological tests using S-LPS, 9, 10, and 23. The best strain for developing a brucellosis vaccine is B. abortus RB51, a rough strain without the disadvantages of the S19 and Rev-1 vaccines. Studies have also demonstrated the immunogenicity of the OMPs of the B. abortus RB51 strain. However, each one alone is not an effective immune system inducer. When used together, they exhibit a synergistic effect in the vaccine. All 11 OMPs were utilized in this study 19,27,28.
SBA is superior to the ELISA method because ELISA measures the antibody production that can only be achieved by a vaccine. Nevertheless, SBA, antigenicity and the serum bactericidal titers against bacteria can be achieved at the same time, as desired. SBA was the main method for testing vaccine safety against Meningitis, and could likely be used to observe the vaccine immunity for brucellosis.
Even though Brucella is an intracellular bacterium, since the newly developed vaccines are killed, it will not be a problem against the intracellular bacteria. Moreover, by the application of both LPS and the OMPs of B. abortus, the humoral and cellular immune responses to vaccines would be significant. The colony counts of mice's spleen demonstrated the effectiveness of this vaccine compared to the control plate. Thus, indications are that the use of these two antigens may be useful in vaccine development.
Compared to the S19 vaccine, this vaccine did not show any sign of abortion. Bioassay in mice and rabbits did not show recurrence. Moreover, this vaccine does not have the risk of transformation of rough strains to soft strains. There are risks of using cattle brucellosis vaccine in humans; however, by using the combination of LPS and the OMPs of Brucella, it will be cleared.
Conclusion :
The study results show that the vaccine induced a T-cell-mediated response through LPS. The OMPs in the vaccine acted as a carrier for the LPS and enhanced the immune response to LPS. Despite concerns, the hope is that production of meat and dairy products could be increased due to the economic damage caused by brucellosis in livestock. Since conjugation of two or more molecules can lead to better immunogenicity in humans or animals, further studies in the future could be done on the evaluation of the immunity caused by the conjugation of these two antigens by SBA.
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Acknowledgement :
We would thanks to the Islamic Azad University Tehran-North Branch, Tehran, Iran.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest :
The authors declare no conflict of interest.
Figure 1. 1) The mean number of colonies per spleen for each group. 2) The number of colonies combined vaccine group OMP+ LPS.
|
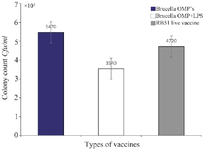
Figure 2. The first column on the left side indicates the spleen colony count for pure Brucella OMP's. The second column in the middle is the spleen colony count for the Brucella OMP+LPS. The column on the right is the spleen colony count done in comparison done with the current RB51 live vaccine.
|
Figure 3. Percentage of subunit vaccine protects against OMP+LPS vaccine RB51: 1) The immunogenicity of combine vaccine: OMP+ LPS (%87.71). 2) The percentage of live vaccine immunogenicity RB51 (% 57.14).
|

Table 1. Efficacy of the combined vaccine vs. the RB51 live vaccine
Bactericidal antibody OMP+LPS vaccines combine the first and second injection. T2: Combine vaccine injection, L: Suicide live vaccine strain RB51 title
|
|