Exploring the Potential of In vitro Maturation (IVM) of Oocytes: Indications, Applications, and Treatment Protocols
-
Torkashvand, Hossein
-
Department of Anatomical Science, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
-
Endometrium and Endometriosis Research Centre, Hamadan University of Medical Sciences, Hamadan, Iran
-
Shabani, Ronak
-
Reproductive Sciences and Technology Research Center, Department of Anatomy, Iran University of Medical Sciences, Tehran, Iran
-
Amiri, Iraj
-
Endometrium and Endometriosis Research Centre, Hamadan University of Medical Sciences, Hamadan, Iran
-
Darakhshan, Roya
-
Endometriosis Research Center, Iran University of Medical Sciences, Tehran, Iran
-
Maleki, Behnam
-
Infertility Center, Department of Obstetrics and Gynecology, Mazandaran University of Medical Sciences, Sari, Iran
-
 Mehdizadeh, Mehdi
Reproductive Sciences and Technology Research Center, Department of Anatomy, Iran University of Medical Sciences, Tehran, Iran, Tel: +98 9121233065; Fax: +98 21 88622689; E-mail: mehdizadeh.m@iums.ac.ir
Mehdizadeh, Mehdi
Reproductive Sciences and Technology Research Center, Department of Anatomy, Iran University of Medical Sciences, Tehran, Iran, Tel: +98 9121233065; Fax: +98 21 88622689; E-mail: mehdizadeh.m@iums.ac.ir
Abstract: This review addresses the current understanding of In Vitro Maturation (IVM) treatment, including indications and effective treatment protocols influencing oocyte developmental competence. A comprehensive literature search was performed to gather relevant studies, clinical trials, and reviews related to IVM. Databases such as PubMed, MEDLINE, and pertinent medical journals were searched. The selected literature was analyzed and synthesized to offer a comprehensive overview. IVM has emerged as a promising technique for inducing maturation in immature oocytes across various developmental stages. Its applications extend to areas utilizing In Vitro Fertilization (IVF), gaining traction as a treatment option for Polycystic Ovary Syndrome (PCOS) and fertility preservation in cancer patients. Recent advancements have led to improved global pregnancy rates, resulting in successful births. IVM also holds potential in reducing risks associated with conventional IVF, including ovarian hyperstimulation syndrome and multiple pregnancies. Despite these advantages, IVM adoption in clinical practice remains limited. Ongoing research aims to refine therapeutic protocols and expand clinical indications. IVM holds promise in assisted reproductive technology, spanning applications from cancer patient fertility preservation to addressing PCOS. Enhanced pregnancy rates highlight efficacy, while risk reduction compared to IVF underscores its importance. Further research is needed for optimal use across patient groups, emphasizing protocol refinement and expanded applications.
Introduction :
In the lead-up to potential fertilization, the follicular phase of the menstrual cycle involves the release of oocytes from pre-ovulatory follicles 1. Although the initial success of In Vitro Fertilization (IVF) occurred in a natural cycle 2, current IVF methods typically use gonadotropins to stimulate multiple follicle development, as it correlates with higher pregnancy rates 3. Traditional IVF involves pretreating women with Gonadotrophin Releasing Hormone analogue (GnRH) followed by Human Menopausal Gonadotrophin (HMG) stimulation, but this can lead to Ovarian Hyperstimulation Syndrome (OHSS), especially in hypersensitive individuals 4–6. The drawbacks of gonadotrophin stimulation include uncertain negative repercussions and the expense of prolonged GnRH and Follicle-Stimulating Hormone (FSH) pretreatment 7.
The emergence of In Vitro Maturation (IVM) technology offers a promising alternative, enhancing the efficiency of natural or mild stimulation IVF. In IVM, both immature oocytes from small follicles and mature oocytes from naturally or minimally stimulated cycles are harvested, increasing the total oocyte yield and the likelihood of successful pregnancies 8. This approach eliminates the need for in vivo hormone injections, addressing concerns related to safety, simplicity, cost-effectiveness, and the avoidance of OHSS, making IVM increasingly appealing to reproductive specialists 9,10. The objective of this review article is to provide a comprehensive analysis of the clinical practice of IVM, including its indications, clinical applications, and treatment protocols. By critically evaluating the available literature on IVM, this review aims to provide valuable insights for clinicians and researchers, and identify areas for further research and improvement in this field.
Definition of oocyte IVM: Oocyte IVM is defined biologically as the process of isolating immature oocytes in the Germinal Vesicle (GV) phase from antral follicles and cultivating them in appropriate culture conditions so they can develop to the Metaphase II (MII) phase in vitro 11,12. Nevertheless, the biological concept of the IVM method for immature human oocytes is totally distinct from its clinical connotation. Variations exist in the sources of immature oocytes, the methods utilized to stimulate ovulation, and the timing of oocyte retrieval across different studies 13. These elements might result in the circumstance when the clinically retrieved immature oocytes are not in the GV stage. As a consequence of using human Chorionic Gonadotropin (hCG) to trigger ovulation before retrieving immature oocytes, some of those oocytes obtained in a clinical setting may have undergone Germinal Vesicle Breakdown (GVBD) or progressed into the MI stage 14. Despite having already initiated IVM, immature oocytes that have progressed to the MI stage require in vitro culture and maturation to complete their maturation process. Accordingly, it is imperative for the clinical definition of IVM treatment to account for the in vitro culture of immature oocytes at both the GV and MI stages.
There's a new viewpoint of thought that says follicle size at the time of oocyte collection ought to be the deciding factor in clinical definitions of IVM with immature oocytes 13. Nonetheless, this definition lacks complete scientific support because the size of follicles throughout the stimulation cycle cannot be used to fully establish the meiotic status of oocytes 11,15. Furthermore, the maturation rates and potential of immature oocytes gathered from various clinical sources to develop into viable embryos and result in successful live births can vary significantly 16. As a result, it is important to evaluate how the effectiveness of IVM for clinical and research purposes can be influenced by the use of various sources of immature oocytes.
Medical indications and therapeutic applications: IVM was originally employed in women who had Polycystic ovary syndrome (PCOS) or severe OHSS during prior IVF treatments. However, as that recently, the scope of IVM usage has broadened to cover nearly all infertility cases, it can now be viewed as a viable alternative. There are several indications that IVM may be suited for, including:
- PCOS 17
- Prior unsuccessful IVF efforts 18
- Previous occurrence(s) of OHSS 10
- Urgent oocyte retrieval due to cancer (estrogen sensitive tumors) 19
- Oocyte retrieval from ovarian tissue prior to vitrification 18
- Poor responders 20
- IVM for IVF cycle rescue 21
- Resistant ovarian syndrome 22
- Preimplantation Genetic Screening (PGS) and Preimplantation Genetic Diagnosis (PGD) 23.
Initially, assisted reproductive technologies, such as IVM, were utilized in clinical settings as an alternative therapy for patients with PCOS 24,25. According to Trounson et al, immature oocytes obtained from IVM cycles maintain their ability to develop in vitro, suggesting a novel treatment option for infertile PCOS patients 26. Numerous researches have since centered on the application of IVM for other indications. Child et al examined the effects of IVM on PCO, PCOS, and unstimulated normal ovaries and came to the conclusion that all three groups' hCG priming had a high potential for maturation, fertilization, and development 27. Seok et al investigated the influence of Anti-Mullerian Hormone (AMH) on individuals with PCOS who favored IVM as the preferred treatment and concluded that AMH was a useful indicator of clinical outcomes in such patients 28. Gremeau et al evaluated 194 PCOS-afflicted women to determine the effectiveness of IVM as instead of traditional IVF and found that IVM was safer, easier to use, and prevented the dangers of OHSS subsequent to IVF 29. Siristatidis et al conducted IVM studies on patients who had and did not have PCOS. Based on the analysis of 11 different trials, it was found that subfertile women with PCOS could benefit from using IVM as a therapeutic option. The study included a total of 268 patients with PCOS undergoing 328 cycles and another 110 patients with PCOS undergoing 110 cycles. These groups were compared with 440 patients who had received dendritic cells 1. The results of the meta-analysis indicated that IVM is an effective treatment option for women with PCOS who are trying to conceive 30. Yoon et al used IVM-derived oocytes to assess the chance of pregnancy in normo-ovulatory women and found a 17.6% pregnancy rate (9/51 embryo transfers). Research has indicated that in cases where the ovaries are normoresponsive, using IVM can result in successful pregnancies. However, it should be noted that the pregnancy rate associated with this method of treatment is generally low 31.
Luteal phase oocyte picks up has created new opportunities for Assisted Reproductive Technology (ART) and cancer patients who wish to preserve their fertility. Because luteal phase oocyte picks up is feasible and effective. In a study by Demirtas et al, the researchers aimed to evaluate the feasibility of retrieving and preserving IVM oocytes from single women undergoing gonadotoxic treatment for cancer. The study included three women, and the researchers were able to easily retrieve IVM oocytes from their luteal phase ovaries. The oocytes were then vitrified for future use 32. Fadini and colleagues explored the use of IVM in women with normal ovulation and juxtaposed it with conventional IVF. Their findings indicated that conventional IVF exhibited higher success rates compared to IVM. However, they suggested that IVM could serve as a viable alternative intervention for specific conditions 33. Fadini et al in their study of prognostic variables for IVM, looked at the importance of body mass index, baseline FSH and estradiol concentrations, Antral Follicle Counts (AFC), endometrial thickness, and leading follicle size. Endometrial thickness and leading follicle size have been identified as predictive factors for determining the optimal timing of immature oocyte retrieval. Furthermore, estradiol levels, FSH concentration, and AFC have been found to be useful in deciding whether IVM treatment should be initiated 34. In the study conducted by Braga et al, the effectiveness of IVM in stimulated cycles was evaluated in 440 patients who were poor responders. The MII-associated immature oocytes were split into two groups and embryos obtained from matured oocytes with rescue spontaneous were added to embryos obtained from matured oocytes in vivo in poor responder patients. In their study, they found that the inclusion of such embryos in poor responder patients led to an increase in the number of transferred embryos and a decrease in cancellation rates; however, there was no significant effect on clinical outcomes 35.
The two most prevalent indications for IVM are PCOS and OHSS, but it can also be utilized in rare circumstances for fertility preservation and instances of Resistant Ovary Syndrome (ROS) 19,36,37. Since ROS is an unusual and intricate clinical presentation, conventional IVF is insufficient for obtaining an adequate number of mature oocytes through Transvaginal Oocyte Aspiration (TVOA), because their follicles are unresponsive to exogenous FSH and their oocytes cannot mature in vivo 22. To achieve pregnancy, ROS patients usually have to accept egg donation. With improvements to IVM in recent years, IVM has become a reliable option for the treatment of this uncommon situation 38. Michaël Grynberg and colleagues documented the initial instance of pregnancy and successful live birth in a patient with ROS using IVM 39. Subsequent to this research, Yu Li et al and C. Flageole et al each presented two additional cases of ROS, wherein pregnancy and live birth were accomplished through IVM 36,40. In a more recent study, Galvao et al identified nine ROS patients and administered IVM treatment, resulting in a live birth rate of 16.7% per initiated cycle and 33.3% per patient 41. Hence, utilizing IVM with patients' own oocytes, as opposed to egg donation, emerges as a meaningful approach for individuals with ROS 42.
According to Lindenberg 43, IVM was also employed for cancer patients, those who had low response rates, and regularly menstruating women. IVM papers published prior to 2009 had low implantation and pregnancy rates, but following Pak et al publication 44, the outcomes were comparable with IVM. IVM may serve as a valuable option for fertility preservation in cancer patients, particularly those without ovarian stimulation and no delay in cancer treatment. A recent case reported a successful pregnancy resulting from cryopreserved embryos obtained from IVM oocytes after oophorectomy in an ovarian cancer patient 45.
With the increasing use of oncofertility treatments, which aim to preserve fertility in cancer patients, it becomes crucial to offer the quickest and least invasive method of fertility preservation. This is particularly relevant for patients with PCO morphology as an additional diagnosis, as they are likely to be the most favorable candidates for successful outcomes 46. As a result, IVM is a good solution for these women since it removes the possibility that OHSS may cause a delay in oncology therapy. In situations where follicular development is abnormally high would and commonly cause an IVF cycle to be cessation, an IVF cycle can be started 47 and then switched to IVM treatment. It has been demonstrated that this technique is effective for storing oocytes and embryos for future fertility 48 and presents a method that might be used for purposes other than oncofertility therapy. Moreover, there are cases where standard IVF is not feasible for patients with particular clinical presentations. In situations where the duration of cancer treatment is a critical factor, IVM therapy can be immediately initiated at any stage of the cycle without requiring stimulation. This approach helps avoid the need to delay chemotherapy, radiation therapy, or surgery. The typical course of therapy for women with estrogen-sensitive malignancies is lowering blood oestradiol levels by giving an aromatase inhibitor or prescribing tamoxifen 49. Nevertheless, IVM could potentially be an appropriate choice for these individuals given that IVM patients continue to have low levels of circulating estrogen and it has been demonstrated that doing so is both secure and successful for those with estrogen-sensitive breast cancer 50. Eventually, women who require pelvic radiotherapy or oophorectomy due to cancer may benefit significantly from IVM treatment, making it the most encouraging area of IVM application. Several case studies have reported positive outcomes using IVM culture after ex vivo immature oocyte harvest following an of these cases resulting in the successful delivery of a healthy baby 45.
IVM could be a good choice for patients whose conventional IVF failed. Gulekli et al 23 women who attempted conventional IVF but failed and switched to IVM without ovarian stimulation were evaluated. While just one pregnancy was achieved through IVM, and that one didn't result in delivery, led the research team to conclude that IVM could be a valuable technique for individuals who have had unsuccessful conventional IVF 51. IVM may be a beneficial alternative in situations where oocyte maturation is problematic, even though it may not be a typical indication for this technique. Hatirnaz et al reported a case where a patient with real Empty Follicle Syndrome (EFS) utilized IVM to mature oocytes retrieved from her. However, due to her partner's azoospermia, only a small number of sperms were produced during the microsurgical Testicular Sperm Extraction (micro-TESE) technique, and only one embryo was transferred on day two with a negative pregnancy test 52. Edward discussed new approaches that could potentially replace traditional IVF, including natural-cycle IVF, minimal-stimulation IVF, and IVM, and placed a particular emphasis on IVM. The author reviewed a substantial amount of data on the genetics and biochemistry of IVM oocytes, and analyzed research papers that provided insights into future possibilities. The author suggested that new follicular growth could potentially be achieved using bone marrow or stem cells obtained from a small blood sample, in both children and adults 53. IVM's role in the IVF treatment plan is not clearly defined, and its classification as either an essential component or simply a laboratory technique needs clarification. In other words, it is not yet clear whether IVM should be considered a fundamental part of IVF or whether it should only be evaluated as a laboratory-based technique.
The potential repercussions of IVM on the chromosomal arrangements and spindle structure of immature oocytes are a subject of concern. Research, using PolScope and immunohistochemical staining for alpha tubulin and chromatin evaluation, has suggested that while supplementing the culture media may enhance maturation rates, it does not necessarily guarantee improved spindle and chromosomal alignment 54. The maturation of oocytes involves sequential but independent processes of nuclear and cytoplasmic maturity. Culture-related conditions have been identified as potential influencers, particularly on cytoplasmic maturation, potentially leading to abnormalities or increased aneuploidy rates 55. A noteworthy study conducted at McGill University compared aneuploidy rates between 6 IVM cycles and 30 IVF cycles using Fluorescence In Situ Hybridization (FISH) analysis. The results revealed similar aneuploidy rates in both groups 56. Therefore, one of the appropriate solutions in the field of IVM, PGS or Preimplantation Genetic Diagnosis (PGD) holds promise. Notably, the first healthy baby resulting from PGD for chromosomal translocation was reported in an IVM cycle 57. The implementation of PGD in IVM has the potential to eliminate developmentally incompetent and aneuploid embryos, thus potentially improving implantation and pregnancy rates, ultimately leading to healthier deliveries.
The effective handling of women displaying suboptimal responses to traditional ovarian stimulation poses an ongoing challenge. Limited data is available on the utilization of IVM protocols for individuals with poor responses. Some investigators have examined whether the implementation of embryo transfers involving rescued IVM-derived embryos might enhance clinical outcomes for patients classified as poor responders during ovarian stimulation 58. In a case study, Liu et al documented three instances of pregnancy (including two successful live births and an ongoing pregnancy) among 8 individuals classified as poor responders. These patients had undergone the IVM of immature oocytes, originating from stimulated IVF cycles before cycle cancellation 59. Certain scholars propose that the use of natural cycle IVF/IVM could yield more favorable results for patients who have been unsuccessful in stimulated cycles, especially those categorized as poor responders 60. In a case study, the authors detailed three instances of pregnancies in individuals with suboptimal ovarian response by integrating natural cycle IVF with IVM of immature oocytes. They proposed that the use of natural cycle IVF/IVM might present a viable alternative for women with poor responses when traditional ovarian stimulation cycles prove ineffective 20. They contended that the retrieval of a greater number of oocytes in natural cycle IVF/IVM cycles, as opposed to natural cycle IVF alone, has the potential to optimize treatment efficacy.
Establishing standard protocols for improved IVM efficacy
Patient Stimulation and Hormonal Priming: The emergence of numerous clinical and laboratory IVM protocols has generated a lot of disagreement and discussion in the literature 11. Nevertheless, the conventional definition of clinical IVM, which is widely acknowledged, is the maturation of immature Cumulus Oocyte Complexes (COCs) obtained from antral follicles that advance from the GV stage to MII stage in vitro over a period of time specific to each species 13 (Figure 1A). The administration of gonadotropins to the patient with the goal of stimulating their antral follicles to generate oocytes with the greatest possible developmental potential (stimulating with FSH) or increasing the percentage of MII oocytes (through the administration of a bolus of hCG before oocyte retrieval) forms the basis of the majority of IVM theme changes.
In other words, the laboratory part of IVM entails collecting and cultivating whole COCs for a period of time that is anticipated to produce MII oocytes. Following that, mature oocytes and the resulting embryos are managed the same manner they would be during a standard IVF cycle. Commonly, COCs are cultivated in complicated tissue culture-type media with the addition of a protein source and hormones (such as FSH+/hCG), in the presence of oxygen. Therefore, it is important to note that there are three basic known clinical IVM laboratory instructions 17, the selection of which one to use is partially determined by the patient's clinical status prior to oocyte retrieval (Figures 1A and 1B). Another factor to take into account is the rescue IVM of GV oocytes from conventional IVF cycles 61 (Figure 1C), although this is not regarded as a clinical IVM operation due to its unorthodox and unrecommended approach 62.
Take note that figure 1 has been adapted from a previously published study carried out by De Vos et al 63. Minor adjustments were implemented to the initial figure in order to enhance its alignment with the focal point of the ongoing research.
Triggering prior to OPU (hCG priming): Due to variations in culture conditions and priming, the outcomes of these protocols are inconsistent and challenging to assess. After oocyte recovery, immature oocytes are subsequently cultivated to obtain MII oocytes in the majority of centers worldwide. This is conventional or standard IVM 64. Conventional IVM can be classified into two systems based on whether HCG priming is carried out prior to oocyte recovery 11. The approach without HCG priming involves the direct recovery and in vitro cultivation of immature COCs (Figure 1A). The with HCG-priming approach involves the administration of HCG 36-38 hr prior COC recovery (Figure 1 A-B). As a result, during retrieval, the oocyte will be obtained in different developmental stages including MII, GVBD and GV. ICSI will be used to treat MII oocytes directly, whereas GVBD and GV oocytes will first undergo IVM before being fertilized by ICSI.
According to the theory, hCG may encourage the beginning of oocyte maturation in vivo, accelerate the process of oocyte maturation in vitro, and thus raise the proportion of mature oocytes. In light of this, priming with hCG before immature oocyte collection may increase live birth rates 65. Additionally, it has been found that the administration of hCG can increase endometrial angiogenesis 66, improve endometrial receptivity 67, and aid in blastocyst implantation 68. In IVM, the first effective use of hCG triggering prior to oocyte retrieval was documented in 1999 69. Many centers have since accepted the utilization of hCG triggering in IVM. In IVM, hCG triggering is typically incorporated with a brief period of FSH priming, with the exception of situations when fertility preservation is important 70.
921 PCOS women participated in the biggest trial of IVM employing hCG triggering and FSH priming. The oocyte maturation rate following one IVM cycle was 71%, while the cumulative live birth rate over a period of 12 months was 33.4% 65. A Cochrane study conducted recently, however, showed no compelling proof that hCG triggering prior to oocyte retrieval and IVM would have an impact on clinical pregnancy or live birth rates 71.
The effect of FSH priming: Various protocols, such as the administration of clomiphene citrate, letrozole, or recombinant or urinary FSH, have been employed for Ovarian Stimulation (OS) prior to oocyte retrieval in IVM 72–74. Short-term administration of FSH to the patient (FSH priming) is the most prevalent OS strategy utilized and evaluated for IVM. According to comprehensive animal research, FSH priming improves follicular growth as well as the meiotic and developmental competence of immature oocytes in vivo 75. Wynn et al conducted the first research that showed moderate stimulation with FSH may increase oocyte production and maturation rates 76. In this trial, a 600 IU dose was applied over the course of five days, beginning on day 2 of the cycle. Although there hasn't been agreement on the dosage and time frame for FSH priming for IVM, nonetheless, the usual dose is 150 IU of FSH daily for two or three days, beginning on days two or three of the cycle or following a progestin discontinuation bleed 67,77.
A study by Vitek et al investigated the issue of Estrogen-Suppressed (ES)-IVM as a cutting-edge and successful IVM technique, and they assessed the clinical and laboratory features of ES-IVM. This approach yielded outcomes that were equivalent to those of natural-cycle IVM or FSH-priming IVM, and it may have eliminated the need for gonadotropins to be administered throughout IVM cycles 78. The issue of early-onset estrogen, either alone or in combination with FSH, is highly debated in the field of IVM, as it may inhibit endogenous FSH and have undesirable consequences on the IVM of oocytes. Another intriguing stimulating strategy for IVM is the employing of letrozole to temporarily increase FSH, while reversibly inhibiting the receptors. Rose investigated the use of letrozole in IVM cycles and attained successful pregnancies as well as subsequent deliveries 79. Letrozole is also crucial for cancer patients who want to maintain their fertility and for people who require urgent IVM using a random-start strategy 55.
Simulation of follicle environment: biphasic IVM development: The advent of biphasic IVM, also known as pre-IVM, into clinical practice is considered a major breakthrough in the field of clinical IVM (Figure 1B). Although the notion of pre-IVM has been discussed in animal literature for years, it provides a substantial new avenue for human IVM 80,81. The fundamental tenets of biphasic IVM culture methods are to (a) keep the oocyte in meiotic arrest (the GV stage) in vitro, (b) keep the physical interaction and paracrine signaling pathway of transmission between the oocyte and cumulus cells intact, (c) establish and sustain a condition that permits the oocyte to gain developmental competence over a period of 24 hr during the pre-IVM phase, and (d) promote the resumption and advancement of meiosis under circumstances that resemble the post-LH surge follicular environment (Figure 1B) 63.
According to research by Sanchez et al, biphasic IVM is better than conventional IVM, and it created the media formulation for "capacitation-IVM" (CAPA-IVM), which was employed in later clinical studies 82. In a major RCT that compared the effectiveness of biphasic IVM with standard IVF, participants in the IVM group got 150 IU of hMG daily for only two days, 5.5 times less FSH than those in the IVF group. Although there was just an 8% difference in the live birth rate following the first embryo transfer between biphasic IVM and standard IVF (35 vs. 43%), which demonstrates that biphasic IVM has the potential to reduce the efficacy difference that exists between IVM and IVF, the higher number of usable embryos following standard IVF led to an approximately 19% lower cumulative ongoing pregnancy rate at 12 months after randomization per started IVM cycle than with standard IVF 77.
As the most significant breakthrough in recent years 63, the introduction of this biphasic IVM into clinical practice has been noted 82–84.
Conclusion :
IVM is a technique with a lengthy history that was developed before IVF. The effectiveness of IVM has greatly increased in recent years due to the development of the IVM culture system. This is particularly crucial for PCOS patients, who are at an extremely high risk of acquiring OHSS. Despite IVM's lower success rates than conventional IVF, substantial advancements were made, resulting in an increase in implantation and live birth rates and a significant reduction in miscarriage and early pregnancy loss, primarily due to the advent of freeze-all techniques. IVM is a good substitute for IVF in some illness scenarios (such as PCOS and ROS). On the other hand, IVM has the ability to preserve the reproductive potential of oocytes in developing follicles, which is frequently lost in most fertility preservation clinics. Even though thousands of healthy IVM infants have been produced and IVM has been employed as a therapy with substantial success, it is still viewed as an experimental technology by the community. The potential for IVM to become as popular and widely used as traditional IVF can be realized through the further development of improved and commercially available IVM culture mediums as well as the establishment of more standardized IVM treatment regimens. Finally, to enable wider acceptance of this significant therapy option globally and facilitate its use as a common treatment strategy in clinics, a more standardized approach to IVM treatment protocols is necessary.
Acknowledgement :
We would like to thank the authors of the primary studies reviewed in this article for their contributions to the field.
Conflict of Interest :
The authors have no conflicts of interest.
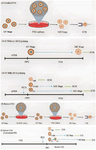
Figure 1. Major IVM protocols. A) The main protocol of IVM, in which GV-stage COCs that are immature are developed to MII in vitro in a single step. A-a) Patients not get an hCG bolus prior Oocyte Pick-Up (OPU). A-b) Patients get a bolus of hCG prior to OPU, +/− prior FSH priming. Only around 10–20% of the oocytes you collect will be in the MII stage; some of them are in the MI stage and majority are in the GV stage. The various phases of meiosis that occur during OPU required distinct types of treatment in the laboratory: MII need to be fertilized on the same day as OPU, while maturing and immature oocytes need IVM culture. B) A biphasic IVM protocol is similar to the standard IVM, except that it includes an extra pre-IVM culture stage. In this protocol, COCs are placed in a meiosis-promoting medium after intentionally arresting immature cumulus-enclosed oocytes for 24 hr. Patients may receive prior FSH priming, but hCG priming is inappropriate due to the need for intact compact COCs in this platform. C) This protocol involves maturing immature oocytes in the GV stage in vitro, which are obtained from conventional IVF cycles after ovarian stimulation and ovulation triggering, often using hCG. In most IVF centers, these oocytes are considered medically useless and are discarded. Rescue IVM oocytes are always cultivated without cumulus cells from GV to MII because these cells are removed after OPU before ICSI.
|
|