The Protective Effect of Crocin on Rat Bone Marrow Mesenchymal Stem Cells Exposed to Aluminum Chloride as an Endocrine Disruptor
-
 Amini, Elaheh
Department of Animal Biology, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran, Tel/Fax: +98 21 88848940; E-mail: elaheh.amini@khu.ac.ir
Amini, Elaheh
Department of Animal Biology, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran, Tel/Fax: +98 21 88848940; E-mail: elaheh.amini@khu.ac.ir
-
Baharvand, Zahra
-
Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Niknejad, Azadeh
-
Department of Cellular & Molecular Biology, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran
-
Tabari, Yasaman
-
Department of Cell and Molecular Biology, University of Science and Culture, Royan Institute, ACECR, Tehran, Iran
-
Shemshadi, Sahel
-
Department of Cell and Developmental Biology, Julius-Maximilians-University, Würzburg, Germany, Germany
Abstract: Background: Mesenchymal Stem Cells (MSCs) have the ability to self-renew and proliferate which gives them healing properties in various tissues. Aluminium chloride (AlCl3) is a chemical compound with harmful effects on health; oxidative stress caused by Aluminium has been reported previously. Crocin, a major component of Crocus sativus (saffron), has antioxidant properties and has shown therapeutic potential. Researchers have been looking for ways to reduce the harmful effects of AlCl3.
Methods: To investigate whether crocin can reduce AlCl3 cytotoxicity, rat Bone Marrow Mesenchymal Stem Cells (BM-MSCs) were isolated, cultured and divided into four experimental groups. The first group was the control, which was untreated cells. The second and third groups were treated with crocin (50, 100, 250, 500 µM) and AlCl3 (20, 25, 30 mM) for 24 hr. The fourth group was pre-treated with crocin (250, 500 µM) for 24 hr and then treated with AlCl3 (20 mM) overnight. Cytotoxicity was assessed using the MTT assay. Mineralization was evaluated by alizarin red staining. Sox-2 and E-cadherin expression were measured using real-time PCR.
Results: The results showed that AlCl3 caused cytotoxicity on BM-MSCs and decreased the mRNA expression of Sox-2 and E-cadherin, which are important for the maintenance of self-renewal and proliferation of BM-MSCs. In contrast, crocin protected the self-renewal characteristic of BM-MSCs by increasing Sox-2 expression and also preserved the proliferative effects on BM-MSCs by upregulating E-cadherin expression (***p≤0.001).
Conclusion: Overall, the study suggests that crocin can protect BM-MSCs from AlCl3-induced cytotoxicity by upregulate Sox-2 expression and E-cadherin expression. This suggests that crocin may be a potential therapeutic agent for the treatment of AlCl3-induced toxicity.
Introduction :
During the past decade, several studies have investigated the effects of natural compounds on the treatment of human diseases such as osteoporosis. Osteoporosis is a type of bone disorder characterized by decreased bone deposition and increased bone resorption. Among these natural compounds, flavonoids act to inhibit bone loss by reducing oxidative stress and promoting osteoblast differentiation 1-3. Saffron is a spice that has been used in traditional medicine for centuries. The active ingredients of saffron have demonstrated a variety of beneficial effects, including anti-proliferative, anti-tumor, anti-inflammatory, anti-oxidant, anti-apoptotic, and osteo-protective effects 4,5.
Crocin is a glycated form of saffron and is a unique water-soluble carotenoid found in saffron. The osteo-protective effects of crocin are thought to be caused by its antioxidative properties. Crocin is a valuable agent against oxidative stress, which can be palliative for the prevention of osteoporosis 6.
Some studies have shown that crocin can be effective in controlling osteoporosis by increasing the number of T cells (Tregs), increasing the level of anti-osteoclastogenic cytokines, and reducing the number of osteoclastogenic Th17 cells 7. Crocin also uses various intracellular pathways, including PI3K/AKT and Wnt/β-catenin signaling pathway 8. Aluminium (Al) as an endocrine disruptor is a toxic agent that is widely present in water, food additives, therapeutic treatments and the environment. Endocrine disruptors are substances that can interfere with various bodily functions, such as fertility, immune function bone development 9. Al can be excreted through urination, but the remaining Al can accumulate in various tissues such as kidneys, liver, heart, blood, brain and bones. Exposure to Al can damage cells and tissues and lead to various diseases such as Alzheimer, osteomalacia and bone disorders 10.
Mesenchymal Stem Cells (MSCs) are present in various tissues, such as adipose tissue, peripheral blood, umbilical cord blood, lung, and bone marrow. MSCs have the ability to differentiate into different cell types, such as osteocyte, chondrocyte, adipocyte, and other lineage 11. MSCs have some important characteristics such as self-renewal and differentiation, which make these cells as a valuable source for healing and inhibiting the progression of diseases such as osteoporosis. These characteristics also make them a promising candidate for regenerative medicine in osteo-related diseases 12.
E-cadherin plays an important role in cell-cell adhesions, which is necessary for the maintenance of cells and tissues integrity 13. Recently, it has been demonstrated that E-cadherin acts as a regulator of pluripotency and self-renewal signaling pathways in stem cells 14. Morphological changes resulting from the interruption of E-cadherin expression can influence cell proliferation and differentiation, which is associated with β-catenin and Wnt signaling pathway 15. One of the modulators of Wnt/β-catenin signaling in development and disease is Sox transcription factors. SOX2 is a core gene that forms a key network essential for the pluripotency and plays a distinct role in maintaining the self-renewal capacity and multipotency of MSCs 16. Therefore, in this study, we investigated cytotoxicity of AlCl3 (Aluminium chloride) and the protective effects of crocin against AlCl3-induced cytotoxicity in rat Bone Marrow Mesenchymal Stem Cells (BM-MSCs) in vitro.
Materials and Methods :
Rat BM-MSCs isolation and culture: All protocols used in this study were approved by the ethics committee of Kharazmi University (Ethical code: (IR.KHU.REC.1399.013). In this study, male Wistar rats (100-120 g) were euthanized with intraperitoneal injection of ketamine (360 mg/kg) and xylazine (30 mg/kg) followed by cervical dislocation. The lumber region to toes were shaved, skin was incised, and the tibia and femur of both legs were exteriorized as described earlier 17. The femur and tibia of both legs were placed in petri dish containing PBS. The metaphysis region of both bones was cut, then a needle inserted into the medullary cavity of bone marrow and flushed out using DMEM (Dulbecco's Modified Eagle Medium) into a 15 ml centrifuge tube. The suspension was then centrifuged for 5 min at 2000 rpm to concentrate the cells. The supernatant was decanted and 5 ml of complete media consisting of 85% DMEM (Bio-idea, Iran), 10% Fetal Bovine Serum (FBS, Bio-idea, Iran), and Penicillin/Streptomycin (100 U/ml) (Bio-idea, Iran) was added to the tube and pipetted. After 72 hr of seeding, non-adherent cells removed by decanting the media. Every 3 or 4 days, the media was replaced until the flask achieved 80-90% confluency. Then, Trypsin-EDTA solution (0.25% Trypsin and 1mM EDTA) was added to passage the flasks. The MSCs were incubated at 37ºC in a humidified atmosphere of 5% CO2 incubator. To be applied in this experiment, BM-MSCs were used at the 3rd-4th passages. Then, four experimental groups were considered. Untreated cells (control) were considered as the first group. In the second and third groups, BM-MSC were treated with various concentrations of crocin or AlCl3 for 24 hr. In fourth group, the cells were pre-treated with various concentrations of crocin for 24 hr, then treated with AlCl3 at IC50 concentration.
Cell cytotoxicity assay: To determine the non-toxic concentration of crocin (Puyesh Darou Sina, Iran) and the IC50 concentration of AlCl3, the MTT assay was performed. In brief, BM-MSCs were plated in 96-well plates at a density of 5×103 cell/well 24 hr ahead of treatment. Untreated MSCs were considered as control. BM-MSC were treated with various concentrations of crocin (50, 100, 250, and 500 µM), AlCl3 (20, 25, and 30 mM) or pre-treated with crocin (50, 100, 250 and 500 µM) and then treated with AlCl3 (20 mM) for 24 hr, in the experimental groups 2 to 4. Each treatment was repeated at least three times. After 24 hr of treatment, MTT solution (0.5 mg/ml) (Sigma, USA) was added to the wells for 3 hr at 37ºC. The medium was the aspirated, and the formazan crystals were dissolved in dimethyl sulfoxide (DMSO, Merck, Germany). Finally, the absorbance was read at 570 nm using iMark™ Microplate Absorbance Reader (Bio-Rad, USA).
The effect of crocin on mineralization of BM-MSCs exposed to AlCl3: To evaluate protective effect of crocin on rat BM-MSCs exposed to AlCl3, the mineralization of BM-MSCs was investigated. In this assay, BM-MSCs (passage 3) were cultured at 2×105 in 6-well plates. The cells were pretreated with crocin (50, 100, 250, and 500 µM) and then subjected to AlCl3 (IC50 value: 20 mM) for 24 hr. The treated and untreated BM-MSCs were also cultured in osteo-differentiation medium containing DMEM supplemented with 10% FBS, 50 µM ascorbate acid, 10 mM β-glycerol phosphate and 10 nM dexamethasone incubated for 21 days.
After the treatment period, the cells were rinsed twice with PBS and fixed in 70% ethanol for 20 min. The calcium deposits were then determined by Alizarin red staining (5 mg/ml in PBS; pH=4.1-4.3). Then, the calcium deposits were evaluated under inverted microscopy (Olympus, Japan).
RNA extraction, cDNA synthesis, and Real-time PCR: In this study, the effect of crocin pre-treatment (250, 500 µM) on BM-MSCs exposed to AlCl3 (20 mM) on the transcription level of Sox-2 and E-cadherin of BM-MCSs was evaluated by real-time PCR. First BM-MCSs (2×106) were seeded in T25 flasks overnight. Then, the cells were exposed to crocin (250, 500 µM) for 24 hr. After pre-treatment with crocin, BM-MSCs were treated with AlCl3 (20 mM) which has a 50% lethal concentration (IC50) for overnight. Total RNA was extracted from untreated and treated cells using total RNA extraction kit (Parstous Biotechnology, Iran) according to the manufacturer’s instructions. Then, the concentration of RNA was determined using a NanoDrop (Thermo Scientific, USA) and cDNA was synthetized from extracted mRNA for each sample using an easy cDNA synthesis kit (Parstous biotechnology, Iran) with the aid of oligo dT primers. Finally, to compare the expression of target genes in the treated groups with the control, real-time PCR was performed using SYBER Green PCR master mix (Takara, Japan) in a Corbett research rotor-gene RG6000 real-time rotary PCR (Corbett, Australia). The results were normalized to the expression of the housekeeping gene, which in this study was GAPDH. The specific forward (F) and reverse (R) primers (5՜-3՜) sequences for real-time PCR were listed in table 1. Finally, the data were analyzed using the ΔΔCt method 18.
Statistical analysis: Statistical analysis was performed using one-way ANOVA followed by post hoc comparisons. One-way ANOVA was used to compare more than two groups. The differences between groups were considered statistically significant at p<0.05*, p<0.01**, and p<0.001***. The SPSS software 22.0 was used for statistical analyses. Results are expressed as the Mean±SEM.
Results :
In vitro expansion of rat bone marrow MSCs: The isolation and in vitro expansion of BM-MSCs were performed. The characterization of rat BM-MSCs was reported in our previous study 17. The proliferative state of BM-MSCs in culture medium containing DMEM with 10% FBS prior to sub-culturing was observed using an inverted microscope (Figure 1).
Effect of crocin on AlCl3-induced cytotoxicity in BM-MSCs: In the current investigation, the MTT assay was performed to determine the proper concentration of crocin that can reduce cytotoxic effects of AlCl3 on BM-MSCs and cause a significant decrease in cell death compared to the control (untreated cells). The IC50 concentration of AlCl3 was also determined. The obtained data revealed that the cell viability of the BM-MSCs was decreased when cells were exposed to AlCl3 at concentrations of 20 mM, 25 mM, and 30 mM for 24 hr. Among these three doses of AlCl3, the 20 mM concentration decreased cell viability to approximately 50% (p<0.001***) compared with untreated cells, and therefore, had a significant cytotoxic effect on rat BM-MSCs (Figure 2A). Furthermore, treatment of MSCs with 50, 100, 250, and 500 µM concentrations of crocin could reduce cell death in a dose-dependent manner (Figure 2B). In addition, the cytotoxicity in the pre-treatment group of BM-MSCs with various concentrations of crocin (250, and 500 µM) and AlCl3 (20 mM) was assessed using the MTT assay. The result showed that crocin (250 µM) decreased AlCl3 cytotoxicity by almost 40% compared with the control, which suggests that crocin at dosage of 250 µM exerted a higher survival rate than cells exposed to 500 µM crocin (Figure 2C).
Effect of crocin on mineralization of BM-MSCs exposed to AlCl3: To assay mineralization induced by crocin on rat BM-MSCs exposed to AlCl3, Alizarin red staining was utilized. As shown in figure 3, exposure to AlCl3 (20 mM) disrupted the shape of BM-MSCs and the mineralization process. Meanwhile, calcium nodules were increased in a dose-dependent manner in pretreated BM-MSCs with crocin, illustrating an elevation of mineralization compared to the control (untreated cells).
On the other hand, pre-treatment with crocin in a concentration of 250 µM (p≤0.001***) and 500 µM (p≤0.01**) resulted in a significantly larger calcium deposit in the treated groups that were pre-treated with crocin and exposed to 20 mM of AlCl3. Therefore, pre-treatment with crocin (250, 500 µM) resulted in osteogenesis, which is characterized by significant mineralization following AlCl3 treatment.
Effect of crocin on Sox-2 and E-cadherin mRNA expression in BM-MSCs: Following treatment of BM-MSCs with AlCl3 in concentration 20 mM for 24 hr, a significant decline in Sox-2 and E-cadherin mRNA expression (p<0.001***) was observed compared to the control (untreated cells) (Figure 4). However, pre-treatment of BM-MSCs with crocin can mitigate the cytotoxicity of AlCl3, which caused up-regulation in Sox-2 and E-cadherin in BM-MSCs.
Discussion :
In this study, we examined the protective effect of crocin against AlCl3-induced cytotoxicity in BM-MSCs. The obtained data showed that AlCl3 reduced cell viability and consequently the mRNA expression of Sox-2 and E-cadherin, which are important for self-renewal and proliferative effects in BM-MSCs, respectively. The results of our current study indicated that the pretreatment of crocin promoted calcium deposit formation, as hallmark of osteogenesis and reduced the cytotoxic effects of AlCl3 on the self-renewal and proliferative effects of BM-MSCs.
MSCs have the ability to self-renew and differentiate into different lineages, and are considered as a possible treatment strategy for various disorders 19. It is documented that in bone defect disorders, including osteoporosis leads to bone fragility. However, it is also known that MSCs can alleviate bone defect disease by promoting osteoblastic differentiation 20. Therefore, the identification of effective natural compounds that can accelerate the osteogenic differentiation of BM-MSCs and overcome the toxicity induced by some chemical compounds can be promising in osteoporosis.
Phytochemicals such as polyphenols and flavonoids isolated from green tea and blueberry or catechine or carnosine, have exhibited a proliferative effect on BM-MSCs. In addition, it has been revealed that phytochemicals exert a unique function in regulating the proliferation and differentiation of stem cells via signaling pathways such as Runx2, BMP2, and Wnt. Therefore, given the role of these signaling pathways in MSCs proliferation and osteoblast differentiation, herbal compounds have promising therapeutic efficacy in bone defect disorders such as osteoporosis 21.
Wnt signaling has an important role in the self-renewal of stem cells in several tissues. Wnt and β-catenin signaling in osteoporosis has high potential therapeutic value by stimulating self-renewal and osteogenesis 22,23. MSCs express oct-4, Sox-2, and rex-1 which are stem cell gene markers. Cadherins interact with Wnts, which have a crucial function in MSC biology and other stem cell niches 24. It is known that Wnt signaling regulates MSC biology by maintaining a balance between self-renewal and terminal differentiation 25. E-cadherin regulates pluripotency and proliferation of stem cells. It has been shown that changes in β-catenin levels can disrupt the adherens junction and E-cadherin and thus influence the Wnt pathway 13.
Sox transcription factors, which are essential pluripotency markers, have important functions during the development of stem cells and precursor cells, including mesenchymal stem cells 26,27. Sox proteins can modulate cell type–specific transcription in response to a Wnt signal. In addition, it has been demonstrated that the modulation of Sox proteins is dependent on signaling pathways such as the sonic hedgehog (Shh) and Wnt signaling pathways 26. Previous studies have reported that Al can induce oxidative stress and also affect metabolism and the expression of various genes by binding to the phosphate group of DNA, RNA, and ATP 10,28. It has been documented that Al can affect signal transduction pathways such as phospholipase C activity, which can lead to inhibition of IP3, regulation of calcium release and activation of protein kinase. Previous investigations have indicated that inhibition of the Wnt/β-catenin signaling pathway by Al exposure can impair bone formation in rats 29,30. In addition, Xu et al, in 2018 reported findings that confirmed the destruction of osteocytes by Al, mainly via oxidative stress-related signaling pathway such as increased levels of c-Jun and p-JNK/JNK ratio as well as recruitment of apoptosis factors 10.
In agreement with these studies, our findings showed the destructive effect of AlCl3 on rat BM-MSCs survival and mineralization. It has been shown that some chemicals such as titanium dioxide and ochratoxin A can suppress the survival, proliferation, and differentiation of stem cells 31,32. It has been shown that oxidative stress and increased levels of Reactive Oxygen Species (ROS) suppress MSC proliferation and promote senescence. Further, ROS increase can affect MSCs differentiation capacity, such as promoting adipogenesis and diminishing osteogenesis 33.
On the other hand, it has been confirmed that crocin can suppress cell‐growth and cell invasion by regulating the Wnt‐pathway and E‐cadherin 34. The protective effects of crocin against chronic stress-induced oxidative damage in the rat brain have been previously elucidated. With regard to its antioxidant properties, crocin may be useful against chronic stress-induced oxidative damage by decreasing MDA level as well as increasing GPX, GR, SOD levels and the total antioxidant capacity. Additionally, the effect of crocin against Acrylamide (ACR) induced toxicity on PC12 cells was evaluated and the findings showed that crocin (10-50 μM) up-regulated Bcl-2, Bax as well as attenuation of apoptosis and ROS generation in PC12 cells 35. Li et al, in 2020, demonstrated that crocin promoted bone regeneration in steroid-induced osteonecrosis of the femoral head (SANFH) rat via acceleration osteoblast differentiation in BM-MSCs 36. In accordance, with these results, our finding showed that crocin acts as a protectant against damage induced by AlCl3 on BM-MSCs.
Conclusion :
Our results demonstrated that pre-treatment of BM-MSCs with crocin can be suggested to reduce the deleterious effects of AlCl3. Overall, crocin as a plant-derived compound, has the potential to overcome the cytotoxicity induced by AlCl3 and exert a healing function mainly by increasing mineralization and modulating the expression of Sox-2 and E-cadherin, which are important for self-renewal and proliferation potential of BM-MSCs.
Acknowledgement :
We thank the staff of the Central Laboratory of Kharazmi University for providing the necessary facilities. This work was supported partially by the research fund for study and research, Kharazmi University (KHU390159172).
Figure 1. The expansion of rat bone marrow mesenchymal stem cells occurs in vitro condition under inverted microscopy (scale bar: 100 µm).
|
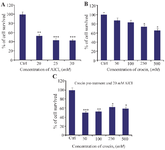
Figure 2. Investigating the effect of various concentrations of crocin, AlCl3 and pre-treatment of crocin and then 20 mM of AlCl3 on cell viability and proliferation of BM-MSCs. A) BM-MSCs were treated with different concentration of AlCl3 ranging from 20, 25, and 30 mM for 24 hr. B) Also the effect of different concentration of crocin (50, 100, 250, 500 μM) on these stem cells for 24 hr was defined with MTT assay. C) Moreover, these stem cells were pre-treated with crocin for 24 hr then treated with AlCl3 (20 mM) showed that crocin 250 µM is proper dose which can decrease the cytotoxicity of AlCl3. Values are Mean±SEM (n=3).
p<0.05*, p<0.01**, p<0.001*** were considered significant between experimental groups with control.
|
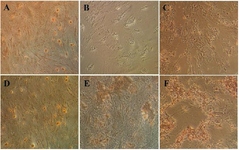
Figure 3. Evaluation of mineralization by Alizarin red staining. The morphology of BMSCs 21 days after treatment period were visualized by inverted microscopy, As shown, suitable concentrations of crocin (250, 500 µM) formed red colored mineral amorphous granules that it was indicating one of the prominent marker differentiations to osteoblasts. A) Control. B) AlCl3 (20 mM) exposed BMSCs cells. C-F) BMSCs pretreated with crocin (50, 100, 250, 500 µM) plus exposure to AlCl3 (20 mM). Magnification× 200.
|
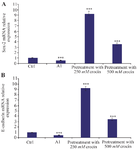
Figure 4. Effect of crocin on mRNA expression of Sox-2 and E-cad induced by AlCl3 treatment of BM-MSCs for 24 hr. A) Here, the impact of pre-treatment with 250 and 500 µM concentrations of crocin and 20 mM AlCl3 on mRNA expression of Sox-2 in BM-MSCs was defined by real time PCR. B) Also, real-time PCR was performed to determine the E-cadherin mRNA expression level. Values are Mean±SEM (n=3).
p<0.05*, p<0.01**, p<0.001*** were considered significant between experimental groups with control.
|

Table 1. List of the specific forward (F) and reverse (R) primer (5՜→3՜) sequences for real-time PCR
|
|