Moringa oleifera Docking to Estrogen Receptor α Ameliorates Placental and Brain Damage in Stressed Rats
-
O. Chukwu , Odochi
-
Department of Physiology, Faculty of Basic Medical Sciences, College of Medical Sciences, David Umahi Federal University of Health Sciences, Ebonyi State, Nigeria
-
Department of Physiology, Faculty of Basic Medical Sciences, College of Medical Sciences, Alex Ekwueme Federal University, Ndufu-Alike. Abakaliki, Ebonyi State, Nigeria
-
 Iyare, Cordilia
Department of Physiology, Faculty of Basic Medical Sciences, College of Medical Sciences, David Umahi Federal University of Health Sciences, Ebonyi State, Nigeria, Tel: +23 48131232377; E-mail: cordie2age@gmail.com
Iyare, Cordilia
Department of Physiology, Faculty of Basic Medical Sciences, College of Medical Sciences, David Umahi Federal University of Health Sciences, Ebonyi State, Nigeria, Tel: +23 48131232377; E-mail: cordie2age@gmail.com
-
C.U. Ezimah , Anthony
-
Department of Physiology, Faculty of Basic Medical Sciences, College of Medical Sciences, Alex Ekwueme Federal University, Ndufu-Alike. Abakaliki, Ebonyi State, Nigeria
-
E. Okorocha , Albert
-
Department of Physiology, Faculty of Basic Medical Sciences, College of Medical Sciences, Alex Ekwueme Federal University, Ndufu-Alike. Abakaliki, Ebonyi State, Nigeria
-
G. Konyefom, Nwaeze
-
Department of Physiology, Faculty of Basic Medical Sciences, College of Medical Sciences, Alex Ekwueme Federal University, Ndufu-Alike. Abakaliki, Ebonyi State, Nigeria
-
P. Igwe, Nancy
-
Department of Anatomy, Faculty of Basic Medical Sciences, College of Medicine, Alex Ekwueme Federal University, Ndufu-Alike Abakaliki, Ebonyi State, Nigeria
Abstract: Background: Stress during pregnancy significantly impacts offspring early physiological programming. Herbal remedies are frequently used by pregnant women to enhance their wellbeing. Moringa oleifera Leaf Extract (MoLE) is believed to have both anti-stress and antioxidant properties which can act as a Selective Estrogen Receptor Modulator (SERM) that regulate activities of estrogen, and can have different effects on different tissues. Goal of this study is to compile information on molecular docking analysis of phytochemicals found in MoLE targeting Estrogen Receptor-alpha (ER-α) and assess effects of MoLE administration on dam's and fetal brain tissues and placenta, during gestational stress.
Methods: Phytochemical study of MoLE was determined using Gas Chromatography-Mass Spectrometry. Molecular docking technique was employed to predict aspects of interaction and binding affinities energy of bioactive phytocompounds in protein site of ER-α using autodock tools. 30 apparently healthy pregnant Albino-Wistar rats were randomly placed into 6 groups of 5 rats per group and exposed to Chronic Unpredictable Stress (CUS) protocol for two weeks, as follows: Group I (water and normal rat chow ad libitum), Group II (CUS protocol only), Group III (5 mg/kg body weight/day of MoLE), Group IV (10 mg/kg body weight/day of MoLE), Group V (CUS protocol +5 mg/kg body weight/day of MoLE), Group VI (CUS protocol +10 mg/kg body weight/ day of MoLE).
Results: This study found that 1-Propanol, 3,3'-oxy bis- and 1, 2, 3-Trimethyldiazir-idine are most potent ligands for ER-α among all 41 compounds. Photomicrograph examination of tissues from stressed rats showed mild to severe alterations in histology. Consumption of MoLE during chronic stress showed mild to moderate protective effects.
Conclusion: These findings suggest that 1-Propanol, 3,3'-oxy bis- and 1, 2, 3-Trimethyldiaziridine can be further investigated for development of novel therapeutics.
Introduction :
Prenatal stress exposure particularly under chronic conditions can profoundly impact fetal brain development and subsequent health outcomes 1,2. The complex interplay between psychological, physiological, and hormonal factors during pregnancy 3, underscores the need for comprehensive investigations into protective strategies. Herbal items are typically seen by pregnant women as a secure, natural substitute for prescription medications 4, and they frequently utilize them to enhance their wellbeing or treat non-life-threatening diseases, the prevalence of using Herbal Medicine (HM) during pregnancy varies depending on the consumer's location, ethnicity, cultural traditions, and social standing 5. Moringa oleifera (MO) a traditionally used medicinal plant, has a widespread consumption, particularly among reproductive-age women in Nigeria, and this has prompted interest in its potential health implications 6. Selective Estrogen Receptor Modulators (SERMs) are a class of ligands that can control estrogenic action. They can be either natural or synthetic. These ligands influence certain Estrogen Receptors (ERs) to provide tissue-specific effects in some tissues while inhibiting estrogen action in other tissues 7, and this type of activity has advantageous pharmacological or nutraceutical benefits 8,9. Chalcones, stilbenoids, lignans, and flavonoids such as isoflavonoids, flavones, flavonols, and flavanones are examples of phenolic compounds that are now classified as phytoestrogens 10. Emerging evidence suggests that phytoestrogens, which are plant-derived compounds with estrogen-like properties, may offer potential neuroprotective benefits 11. MO is believed to be rich in diverse phytoestrogens 12,13, while previous studies have highlighted general estrogenic effects of Moringa oleifera Leaf Extract (MoLE) 13,14, specific mechanisms underlying its potential neuroprotective actions remain largely unexplored.
Estrogen Receptor alpha (ER-α), a key mediator of estrogenic effects is expressed in brain regions crucial for cognitive function and emotional regulation 7,15. The intricate balance between ER-α and its interaction with phytoestrogens offer a promising avenue for therapeutic intervention. Moreover, the impact of prenatal stress on ER-α function and the potential modulatory effects of phytoestrogens warrant further investigation.
This study aims to elucidate the molecular mechanisms by which phytoestrogens in MoLE exert neuroprotective effects. By combining molecular docking analysis to identify potential interactions with ER-α and in vivo evaluation of the effects of MoLE supplementation on a prenatal stress condition, we seek to provide novel insights into the therapeutic potential of this plant for mitigating the adverse consequences of prenatal stress on brain development.
Materials and Methods :
Plant collection, identification, and extraction: Fresh leaves of MO were collected early in the morning from a garden in Abakaliki, Ebonyi State, Nigeria. Plant authentication was confirmed by a botanist from the Herbarium Unit, Department of Biological Science, Alex Ekwueme Federal University Ndufu-Alike Ikwo (AE-FUNAI). Collected leaves were shade-dried for two weeks and subsequently ground into a fine powder (particle size <250 µm) using a grinding machine (Miller: model ms-233, China) 13. A standardized Soxhlet extraction method was employed using methanol as the solvent. Two hundred g of the powdered leaves were extracted three times for 48 hr each. The combined filtrates were concentrated using a rotary evaporator at 40°C to obtain a pasty dark green extract, which was stored at 4°C in an airtight labelled container to ensure potency, the yield was calculated 13.
Phytochemical screening and GC-MS analysis: Phytochemical screening was conducted to determine the presence of various bioactive compounds in the MoLE leaf extract. Standard procedures outlined by Trease and Evans 16 were employed to detect alkaloids, saponins, glycosides, flavonoids, phenols, tannins, and steroids. Gas Chromatography-Mass Spectrometry (GC-MS) was employed for the identification and semi-quantitative analysis of bioactive compounds in the extract. The Agilent 6890 gas chromatograph equipped with an HP-88 capillary column was used. The GC-MS parameters included an ionization voltage of 70 eV, oven temperature of 180°C (held for 1 min), an injection volume of 1 µl, and a run time of 15 min. Compound identification was achieved by comparing mass spectra with the NIST11 library. Peak area in the chromatogram was used to estimate relative compound abundance 13.
Molecular docking analysis: To explore the potential binding interactions of phytochemicals from MoLE with estrogen receptors, molecular docking simulations were performed. The crystal structures of the ER-α (PDB ID: 1L2J) were retrieved from the Protein Data Bank. Ligand structures were obtained from the PubChem database and converted to mol2 format using Open Babel 17. Docking simulations were carried out using Vina to predict the preferred binding orientations and affinities of ligands within the receptor binding sites. The binding affinity, represented as docking score, is an estimation of the free energy of ligand-receptor interaction. A more negative docking score indicates a stronger binding affinity. To visualize and analyze protein-ligand interactions, Discovery Studio 2020 was utilized. By comparing the binding modes and interactions of different ligands, insights into structure-activity relationships and potential mechanisms of action can be gained 18.
Ethical approval: The study was approved by the Faculty of Basic Medical Sciences Research Ethics Committee, Alex Ekwueme Federal University Ndufu-Alike, Ebonyi State, Nigeria with code FBMS/EC/AE/1983. This study was carried out in accordance to ARRIVE guidelines.
Experimental design: Thirty mature inbred apparently healthy virgin female Albino-Wistar rats were procured from the Animal House, AE-FUNAI, Ebonyi State. They were acclimatized to their feed (Vital feed®, Nigeria) and water ad libitum for 2 weeks before commencement of the experiment. On the day 1 of pregnancy, the thirty rats were randomly placed into 6 groups of five rats per group as follows:
Group one (Normal Control): received only water and normal rat chow ad libitum throughout pregnancy.
Group two [Chronic Unpredictable Stress–(CUS) Control]: was exposed to CUS protocol only from Gestational Day (GD) 8–GD 21st day.
Group three (Low Dose MoLE): was administered 5 mg/kg body weight/day of MoLE throughout GD 8–GD 21st day.
Group four (High Dose MoLE): was administered only 10 mg/kg body weight/day MoLE throughout GD 8–GD 21st day.
Group five (CUS+Low Dose MoLE): was exposed to CUS protocol +5 mg/kg body weight/day of MoLE throughout GD 8–GD 21st day.
Group six (CUS+High Dose MoLE): was exposed to CUS protocol +10 mg/kg body weight/day of MoLE throughout GD 8–GD 21st day.
Oral gavage was used for all administration, once per day, following exposure to CUS regimen. After 21 days, pups were weaned to tap water and feed ad libitum and they retained the groups of the dams. The male and female pups were housed separately after weaning. The gestational period from GD 8 to GD 21 was selected as it corresponds to a critical period of brain development in rats, analogous to the second and third trimesters in humans, when the brain is highly susceptible to environmental influences. The doses of MoLE (5 and 10 mg/kg/day) were chosen based on previous studies by Chukwu et al 13, demonstrating the safety and efficacy of MoLE at similar doses in animal models. Additionally, these doses were selected to evaluate a dose-dependent response to MoLE treatment by Chukwu et al 13.
Initiation of pregnancy: Observation of the estrus cycles was possible by using light microscopy, and the animals who exhibited two consecutive regular four-day estrus cycles were selected for the study. During the pro-estrus phase, male rats were placed into the cages of female rats at a ratio of 1:2 in order to facilitate mating. The subsequent morning, which was the first day of pregnancy, the presence of spermatozoa in the female rats' vaginal smears served as confirmation of the successful mating event 19,20.
Chronic Unpredictable Stress (CUS) protocol: The stress group of animals were subjected to CUS protocol from GD 8 (which is around the period of onset of organogenesis in rats) to GD 21 (expected day of parturition). Briefly, CUS protocol comprising a variety of stressors was applied in an unpredictable manner, as listed below:
Wet bedding: 300 ml of water was poured on and mixed with 1 L of sawdust bedding.
Cage tilting: the cage was tilted up to 45 degrees with food and water located at the higher top. One overnight period of difficult access to food.
Psychological stress: rats were exposed to a caged cat.
Sleep deprivation: a cylindrical, wooden pedestal (6 cm diameter and 5 cm height) was placed on the floor of the cage opposite to the food/water compartment. The cage was then flooded with tap water 3 cm deep, allowing the animal to stand on the bottom of the pedestal but denying the possibility of sleeping.
Restraint Stress: rats were placed in a 50 ml plastic tube with openings in both sides for breathing, for 6 hr (3 sessions of 2 hr with 30 min gap). One overnight period of permanent light.
Social isolation (SI): is the most replicated stressor within the CUS model, because animals remained single-housed during the implementation of each stressor. These stressors were given subsequently and animals received one stressor everyday which makes each stressor unpredictable for the total course of pregnancy. All stressors lasted for 6 hr/d, except for the sleep deprivation sessions that lasted for 12 hr. Animals under stress groups were exposed to every stressor twice throughout the protocol. The CUS protocol was employed to mimic the unpredictable and uncontrollable nature of human prenatal stress. This model has been widely used in preclinical studies to investigate the effects of stress on maternal and fetal outcomes 13,21.
Sample collection and histological study: The dams were sacrificed by cervical dislocation at GD 20 and following abdominal incision, the foetuses along with the placenta were first removed and placed in a new formaldehyde solution after which the dams brain was also removed and placed in a new formaldehyde solution for 24 hr before being dehydrated using ethanol (70% for 24 hr 90% for 1 hr and 100% for 1 hr) then cleaned in xylene and embedded in paraffin. Coronal sections were cut with a microtome, at 5 μm thicknesses, mounted on glass slides and stained with the routine Hematoxylin and Eosin technique. Examination of slides, morphometric studies, and photomicrography was then done 20.
Data analysis: Data analysis was primarily qualitative in nature, focusing on the identification and characterization of phytochemical constituents through GC-MS analysis, as well as the assessment of molecular docking interactions. Given the exploratory nature of the study and the limited sample size, statistical analysis was deemed inappropriate.
Results :
GC-MS analysis of the MoLE identified forty-one compounds (Table 1), with steroidal fatty acid methyl esters, including hexadecanoic acid methyl ester, as predominant constituents. To explore the potential binding interactions of these compounds with ERs, molecular docking simulations were performed. Among the identified compounds, 1-Propanol, 3,3'-oxybis- exhibited a strong binding affinity to the ER-α with a docking score of -8.8 kcal/mol. Key interactions included hydrophobic (π-alkyl) interactions with ARG A:503 and VAL A:316, electrostatic interactions (π-cation) with LYS A:492, and hydrogen bonding with LEU A:489 and GLU A:443 (Figure 1). Furthermore, 1,2,3-Trimethyldiaziridine demonstrated strong binding affinities to ER-α (-7.8 kcal/mol), characterized by hydrophobic interactions (π-alkyl and π-π stacking) with amino acid residues LEU, ALA, and PHE (Figure 2). Clomiphene, a reference compound, exhibited multiple interaction types with ER-α, including hydrophobic (π-sigma, π-π stacking, π-alkyl) and electrostatic interactions (Figures 3 and 4).
Histopathological examination revealed varying degrees of alterations across different brain regions and the placenta. For the prefrontal cortex: Group II exhibited severe degenerative changes characterized by neuronal loss, prominent microcystic spaces, and focal areas of necrosis (Figure 5). In contrast, Groups V and VI, treated with MoLE, showed milder alterations with reduced neuronal loss and less pronounced microcystic spaces (Figures 6 and 7).
For the fetal brain: Group II displayed severe degeneration with extensive neuronal loss, inflammatory cell infiltration, and tissue loss (Figure 8). MoLE treatment (Groups III-VI) resulted in varying degrees of improvement, with reduced severity of lesions compared to the control group (Figures 9-12).
For the placenta: Group II exhibited severe placental pathology characterized by edema, multinucleated giant cell formation, and hemorrhage (Figure 13). MoLE treatment (Groups III-VI) resulted in milder placental lesions with reduced severity of these changes (Figures 14-17).
Discussion :
Prenatal stress is a well-established risk factor for adverse neurodevelopmental outcomes. The present study findings demonstrate significant histological abnormalities in the prefrontal cortex (Figure 5), fetal brain (Figure 8), and placenta (Figure 13) of the Group II, indicative of the detrimental effects of prenatal stress on brain and placenta structure and function. These observations align with previous studies highlighting the vulnerability and detrimental impact of prenatal stress on developing brain structure and function, as well as placental health 22-24.
Histopathological examination of the prefrontal cortex revealed severe degeneration in the Group II, characterized by neuronal loss, microcystic spaces, and necrosis. In contrast, MoLE-treated groups (Groups V and VI) exhibited milder alterations (Figures 6 and 7). Similarly, MoLE treatment attenuated the severity of histological abnormalities in the fetal brain (Figures 9-12) and placenta (Figures 14-17) compared to Group II. MoLE treatment attenuated the stress-induced histological abnormalities, suggesting potential neuroprotective and placental-protective effects through its modulation of estrogenic signaling pathways. These findings align with previous studies highlighting the beneficial effects of phytochemicals on brain health 11. However, this study provides additional insights into the potential mechanisms of action by demonstrating the binding affinity of 1-Propanol, 3,3'-oxybis- to the ER-α and its potential role in mediating MoLE's neuroprotective effects. The observed neuroprotective effects of MoLE may also involve other mechanisms, such as antioxidant, anti-inflammatory, and immunomodulatory properties, which have been attributed to MO 25.
Estrogen plays a critical role in optimal neurodevelopment and function, and imbalances in estrogen signaling have been implicated in various neurological disorders 9. Prenatal stress can disrupt this delicate balance, potentially exacerbating the vulnerability of the developing brain and placenta. ERs are widely expressed in the central nervous system, and their modulation has therapeutic potential for various neurological disorders. Molecular docking studies identified potential interactions between phytochemicals in MoLE and ER-α. Compounds with strong binding affinities and favorable interaction profiles may act as SERMs, exerting tissue-specific effects.
The molecular docking analysis revealed that 1-Propanol, 3,3'-oxybis- exhibited strong binding affinity to the ER-α with key interactions including hydrophobic, electrostatic, and hydrogen bonding (Figure 1). This compound's interaction with ER-α may contribute to its observed neuroprotective effects 3. While the exact mechanisms underlying these effects require further investigation, it is plausible that modulating estrogen receptor signaling could influence neuronal survival, plasticity, and inflammation. By interacting with ER-α, 1-Propanol, 3,3'-oxybis- may exert estrogenic or anti-estrogenic effects, depending on the specific cellular context and receptor subtype involved. 1,2,3-Trimethyldiaziridine also demonstrated strong binding affinity to ER-α (Figure 2), characterized by hydrophobic interactions (π-alkyl and π-π stacking). This finding suggests that multiple phytochemicals within MoLE may interact with ERs, potentially contributing to the overall therapeutic effects of the extract. Clomiphene, a reference compound, exhibited multiple interaction types with ER-α, including hydrophobic and electrostatic interactions (Figures 3 and 4). Comparison of the binding profiles of 1-Propanol, 3,3'-oxybis- and 1,2,3-Trimethyldiaziridine with clomiphene provides valuable insights into the structure-activity relationships of these compounds and their potential interactions with the estrogen receptor. Further studies are needed to elucidate the precise mechanisms of action and therapeutic potential of these compounds.
While acknowledging the limitations of the current study, it is important to emphasize the novel findings and potential implications of this research. The use of a single animal model and the absence of behavioral assessments restrict the generalizability of these findings to human populations and our understanding of the full spectrum of effects of MoLE. Future studies incorporating diverse animal models and behavioral endpoints would further elucidate the mechanisms underlying MoLE's neuroprotective effects and its potential clinical applications.
Conclusion :
Prenatal stress significantly contributes to adverse neurodevelopmental outcomes, as evidenced by the observed histological abnormalities in the prefrontal cortex, fetal brain, and placenta. This study demonstrates the potential of MoLE in mitigating the detrimental effects of prenatal stress. Molecular docking analysis revealed that 1-Propanol, 3,3'-oxybis- exhibited strong binding affinity to the ER-α, suggesting a potential mechanism for MoLE's neuroprotective effects. These findings contribute to the growing body of evidence supporting the role of phytoestrogens in modulating estrogenic signaling pathways. While further research is needed to elucidate the precise mechanisms underlying MoLE's actions, this study provides a foundation for the development of novel therapeutic strategies targeting prenatal stress-induced neurodevelopmental disorders. The identification of compounds within MoLE with potential ER modulating properties offers promising avenues for future drug discovery and development.
Acknowledgement :
The authors thank the Department of Physiology, Faculty of Basic Medical Sciences, College of Medicine, AE-FUNAI, Ebonyi State, for supporting this study.
Conflict of Interest :
The authors declared no conflict of interest related to this article.
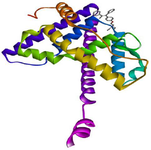
Figure 1. 3D view of the interaction between clomiphene and the binding site of estrogen receptor alpha.
|
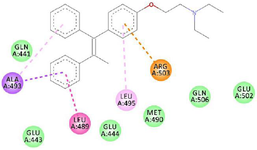
Figure 2. 2D view of the interaction between clomiphene and amino acids in the binding site of estrogen receptor alpha.
|
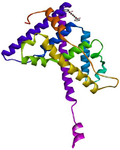
Figure 3. 3D view of the interaction between 1-Propanol, 3,3'-oxybis- and the binding site of estrogen receptor alpha.
|
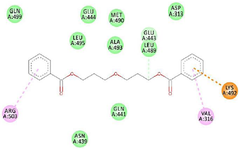
Figure 4. 2D view of the interaction between 1-Propanol, 3, 3'-oxybis- and amino acids in the binding site of estrogen receptor alpha.
|
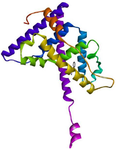
Figure 5. 3D view of the interaction between 1, 2, 3-Trimethyldia-ziridine and the binding site of estrogen receptor alpha.
|
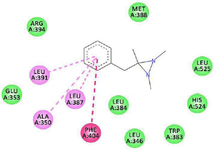
Figure 6. 2D view of the interaction between 1, 2, 3-Trimethy-ldiaziridine and amino acids in the binding site of estrogen receptor alpha.
|
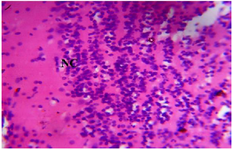
Figure 7. Photomicrograph of Group One (Normal Control) section of the Dam’s Prefrontal Cortex (x400) (H/E) shows normal active neuronal cell (NC) of the Prefrontal Cortex.
|
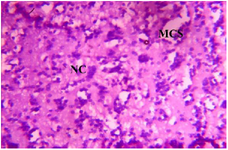
Figure 8. Photomicrograph of Group Two (CUS control) section of the Dam’s Prefrontal Cortex (x400) (H/E) shows moderate to severe degeneration with severe Microcystic Spaces (MCS) moderate focal areas of Necrotic Cells (NC).
|
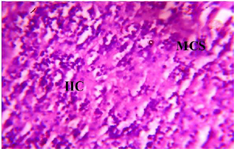
Figure 9. Photomicrograph of Group Three (Low Dose MoLE) section of Dam’s Prefrontal Cortex (x400) (H/E) shows mild Infiltration of Inflammatory Cells (IIC) with Microcystic Spaces (MCS) and active Granular Cells (GC) in some area.
|
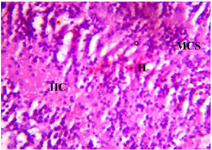
Figure 10. Photomicrograph of Group Four section of the Dam’s Prefrontal Cortex (High Dose MoLE) (x400) (H/E) shows moderate infiltration of Inflammatory Cells (IIC) with Microcystic Spaces (MCS) and mild area of Hemorrhage (H).
|
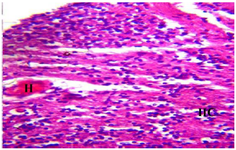
Figure 11. Photomicrograph of Group Five (CUS+Low Dose MoLE) section of the Dam’s Prefrontal Cortex (x400) (H/E) shows moderate to severe degeneration with Infiltration of Inflammatory Cells (IIC) and moderate area of Hemorrhage (H).
|
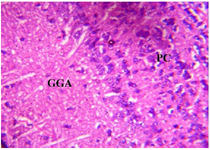
Figure 12. Photomicrograph of Group Six (CUS+High Dose MoLE) section of Dam’s Prefrontal Cortex (x400) (H/E) shows mild Ground Glass Appearance (GGA) and Pyknotic Cells (PC) in some area.
|
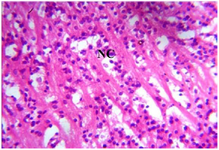
Figure 13. Photomicrograph of Group One (normal control) section of the Fetal Brain Tissue (x400) (H/E) shows Cerebral Cortex with active normal Neuronal Cells (NC).
|
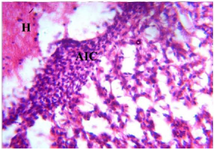
Figure 14. Photomicrograph of Group Two (CUS Control) section of the Fetal Brain Tissue (x400) (H/E) shows severe degeneration with severe Aggregate of Inflammatory Cells (AIC), Focal Area Haemorrhage (H) and severe Loss (L) of Brain Tissue with
non distinct Neuronal Cell outline.
|
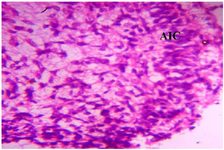
Figure 15. Photomicrograph of Group Three (Low Dose MoLE) section of the Fetal Brain Tissue (x400) (H/E) shows moderate regeneration with mild Aggregate of Inflammatory Cells (AIC).
|
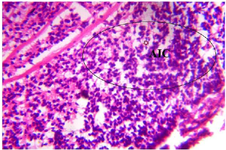
Figure 16. Photomicrograph of Group Four (High Dose MoLE) section of the Fetal Brain Tissue (x400) (H/E) shows mild degeneration with moderate Aggregate of Inflammatory Cells (AIC).
|
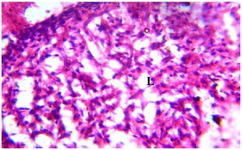
Figure 17. Photomicrograph of Group Five (CUS+Low Dose MoLE) section of the Fetal Brain Tissue (x400) (H/E) shows moderate to severe degeneration with Aggregate of Inflammatory Cells (AIC), Loss (L) of Fetal Brain Tissue with Pyknotic Neuronal Cell.
|
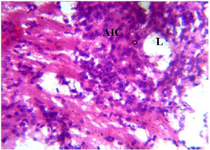
Figure 18. Photomicrograph of Group Six (CUS+High Dose MoLE) section of the Fetal Brain Tissue (x400) (H/E) shows moderate degeneration with mild Aggregate of Inflammatory Cells (AIC) moderate Loss (L) of Fetal Brain Tissue with Pyknotic Neuronal Cell.
|
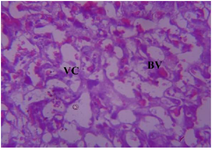
Figure 19. Photomicrograph of Group One (Normal Control) section of the Placenta (x400) (H/E) shows uniform Vascular Channels (VC) with mild Blood Vassetion (BV).
|
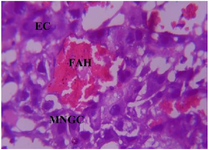
Figure 20. Photomicrograph of Group Two (CUS Control) section of the Placenta (x400) (H/E) show severe degeneration with severe Edematous Change (EC), severe cluster of Multinucleated Giant Cells (MNGC) and severe Focal Area of Hemorrhage (FAH).
|
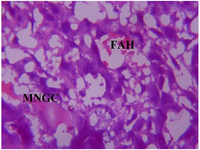
Figure 21. Photomicrograph of Group Three (Low Dose MoLE) of the Placenta (x400) (H/E) shows mild degeneration with moderate cluster of Multinucleated Giant Cells (MNGC) and moderate Focal Area of Hemorrhage (FAH).
|
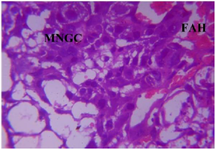
Figure 22. Photomicrograph of Group Four (High Dose MoLE) of the Placenta (x400) (H/E) shows mild degeneration with moderate cluster of Multinucleated Gaint Cells (MNGC) and moderate Focal Area of Hemorrhage (FAH).
|
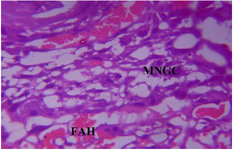
Figure 23. Photomicrograph of Group Five (CUS+Low Dose MoLE) of the Placenta (x400) (H/E) shows moderate degeneration and cluster of Multinucleated Giant Cells (MNGC) with moderate Focal Area of Hemorrhage (FAH).
|
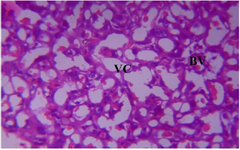
Figure 24. Photomicrograph of Group Six (CUS+High Dose MoLE) section of the Placenta (x400) (H/E) shows moderate Blood Vassation (BV) and uniform Vascular Channels (VC).
|

Table 1. Bioactive compounds of Moringa oleifera leaf extract identified by GC-MS and their Binding affinity to ER-α
|
|