5-Fluorouracil Effectively Depletes Tumor Induced Myeloid Derived Suppressor Cells in 4T1 Mammary Carcinoma Model
-
Ramezani-Aliakbari, Khadijeh
-
Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Department of Pathobiology, Faculty of Veterinary Science, Bu-Ali Sina University, Hamadan, Iran
-
Jalali , Seyed Amir
-
Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Alinejad, Maedeh
-
Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Jeddi-Tehrani, Mahmood
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
 Shabani, Mahdi
Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran, Tel: +98 21 22439970; Fax: +98 21 22439970; E-mail: msshabani@sbmu.ac.ir, msshabani@yahoo.com
Shabani, Mahdi
Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran, Tel: +98 21 22439970; Fax: +98 21 22439970; E-mail: msshabani@sbmu.ac.ir, msshabani@yahoo.com
Abstract: Background: Myeloid Derived Suppressor Cells (MDSCs) are capable of inhibiting both innate and adaptive immune responses and accumulate in the microenvironment of breast tumors. Hence, MDSC depletion by chemotherapeutic agents can improve clinical efficacy of cancer immunotherapy. The effects of 5-FU and doxorubicin agents on MDSC reduction in 4T1 breast cancer murine model were evaluated.
Methods: 5×105 of 4T1 tumor cells were injected into mammary fat pad of BALB/c female mice. Tumor bearing mice were randomly divided into 4 groups: PBS receiving control group, doxorubicin receiving groups at doses of 2.5 and 5 mg/kg, and 5-FU receiving group at dose of 50 mg/kg. Doxorubicin and 5-FU agents were intraperitoneally administrated at three doses with 5-day intervals and five doses for three times a week, respectively. Then, on day 20 post tumor cells injection, spleens and tumors were isolated to determine frequency of CD11b+ Gr1+ MDSCs by flow cytometry analysis.
Results: 5-FU was able to reduce significantly both splenic and interatumoral MDSCs comparing to control group (p=0.0276 and p=0.0067, respectively). Also, Doxorubicin treatment at dose of 50 mg/kg was associated to a significant reduction of splenic MDSCs in comparison to untreated group (p=0.0382). However, only 5-FU injection led to inhibit notably tumor growth in comparison to control group (p=0.0139).
Conclusion: Findings show that 5-FU has inhibitory effects on MDSCs and tumor growth in 4T1 tumor model. So, more investigations are needed to study combination of 5-FU with immune based approaches to enhance the efficacy of cancer therapies.
Introduction :
Myeloid Derived Suppressor Cells (MDSCs) have been identified as a heterogenous population of immature myeloid cells accumulated in different human cancers and also tumor animal models 1,2. Murine MDSCs express CD11b and Gr-1 surface markers and in human, MDSCs are defined by CD14- CD33+ CD11b+ HLA-DRneg/low phenotype 3,4. Also, murine MDSCs are further characterized by expression profile of Gr-1 isoforms (Ly6G and/or Ly6C) and subdivided into two main subsets: granulocytic MDSCs (CD11b+ Ly6G+ Ly6Clow) and monocytic MDSCs (CD11b+ Ly6G- Ly6Chigh) 4. MDSCs are able to inhibit both innate and adaptive immune reactions via their immunoregulatory mediators such as arginase, indoleamine 2,3-dioxygenase (IDO) and oxidative species 5. Overall, MDSCs have a key role in the tumor-induced immunosuppression and so have been investigated as a therapeutic or prognostic marker in clinical trial settings to improve clinical efficacy of immune-mediated interventions or prognosis of many cancers 6,7. To target MDSCs, a variety of agents range from conventional drugs to biologic products were approved for clinical benefit in cancer immunotherapy settings through restoration of immune responses directed tumor antigens 8,9. In addition to direct cytotoxic effects, chemotherapeutic agents can act by reduction of MDSCs frequency in primary tumors and peripheral lymphoid organs resulted in enhancement of anti-tumor responses 10,11. Also, combing of conventional anti-cancer agents with immunotherapy-based approaches has been shown to improve anti-tumor immune reactions in murine models and patients with cancer 12. For example doxorubicin, a neoplastic drug, can effectively eliminate MDSCs accumulated in blood, spleen and tumor from 4T1 tumor bearing mice and foster efficacy of TH1/TH17 lymphocytes -based immunotherapy via induction of NK cells and CTL mediated cytotoxic responses 13. Moreover, several cytotoxic agents such as cyclophosphamide, paclitaxel, gemcitabine, and 5-Fluorouracil (5-FU) have been screened to deplete the tumor- induced MDSCs in EL4 thymoma murine model 14. Among them, only gemcitabine and 5-FU resulted in a major decrease of MDSCs in spleen and tumor bed without significant effects on other immune cells by triggering of apoptotic cell death of MDSCs in vivo 14. In addition to EL4 model, 5-FU injection at a single 50 mg/kg dose has been found a significantly decrease of MDSCs in spleen and lung of 4T1 tumor model 15. Also, it was reported MDSC depletion by gemcitabine augmented anti-tumor effects of tumor vaccines in patients with ovarian and cervical cancers 16. These findings indicate some chemotherapeutic agents including 5-FU and doxorubicin have inhibitory effects on MDSCs from tumor bearing hosts and can be evaluated in combination with cancer immunotherapies. To investigate whether 5-FU and doxorubicin agents are capable of overcoming on MDSCs accumulation in breast cancer, effects of 5-FU and doxorubicin anti-cancer agents at multiple low doses on MDSCs frequency in 4T1 mammary carcinoma model were explored.
Commonly used chemotherapeutics drugs have low cost and known anti-tumor effects comparing to anti-cancer biologic products such as monoclonal antibodies and recombinant proteins. On the other hand, tumor- induced MDSCs are a main inhibitory population counteracting the successful immunotherapy via suppression of anti-tumor immune responses. Thus, targeting of MDSCs along with other immune-based therapeutic approaches can take the benefit from both activation of immune system and weakening of immune suppressive mechanisms. Regarding to MDSCs importance in the tumor immune tolerance and commonly use of chemotherapeutics, the aim of the present study was to investigate effects of 5-FU and doxorubicin agents on MDSCs in 4T1tumor model (Figure 1).
Materials and Methods :
4T1 tumor model induction: Female Balb/C mice (6-8 weeks old) were purchased from Pasteur Institute of Iran (Karaj, Iran) and maintained under standard conditions at temperature of 22±1°C with 50% humidity and 12/12-hr light/dark cycle. 4T1 cell line was purchased from the National Cell Bank of Iran (Tehran, Iran) and then tumor cells were cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% FBS (Gibco, NY, Grand Island, USA). To induce 4T1 tumor model, 5×105 cells were administrated into the mammary fat pad of female mice. One week after cell line injection, mice were checked for formation of palpable mammary tumors. All animal experiments were performed according to guidelines of the Ethical Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1396.874).
Treatment schedule of tumor bearing mice: 4T1 tumor bearing mice were randomly divided into 4 groups (5 mice per group) received following treatments: PBS (control group), doxorubicin (Ebewe, Austria, Unterach) at dose of 2.5 mg/kg, doxorubicin at dose of 5 mg/kg, 5-FU (Ebewe, Austria, Unterach) at dose of 50 mg/kg. Treatments started eight days post 4T1 tumor inoculation in female Balb/C mice and finally sacrificed 20 days after tumor cells injection. Doxorubicin and 5-FU were intraperitoneally injected at three doses with 5-day intervals and five doses for three times per week, respectively (Figure 2). Tumor dimensions were recorded three times a week and tumor volumes were determined by following formula:
Tumor volume mm3=d2 (smallest diameter)×D(largest diameter)2
Twelve days post treatments, tissues including spleens and mammary tumors were isolated to detect MDSCs population by flow cytometry method (Figure 2).
Flow cytometry: To analysis MDSCs population, single cell suspensions from isolated tissues were prepared. Spleens were mechanically disrupted and then filtered by cell strainer 0.7 μm (BD Falcon, Bedford, MA, USA). Lysis buffer (0.8% NH4Cl) added to cell suspensions for 5 min at 4°C to lyse RBCs resident of spleens. Also, tumors were cut into 2–4 mm pieces and then mechanically dissociated using a tissue grinder (Kimble Chase, Rockwood, TN, USA). To evaluate effects of treatments on MDSC population, isolated splenocytes and tumor cells were co-stained with PerCP-conjugated anti-CD11b and FITC-labelled anti-Gr1 antibodies (1 µg/ml) (Biolegend, San Diego, CA, USA) for 30 min at 37°C. FACS analysis was conducted using flow cytometer cell analyzer (BD Biosciences, FACS Lyrics, San Jose, CA, USA) and the data was analyzed by Flow Jo software V7 (Tree Star Inc, Ashland, OR, USA).
Statistical analysis: Statistical analyses were performed by Graph Pad Prism 8.0 software (Graph Pad Software Inc., San Diego, CA, USA) using none parametric Kruskal-Wallis test to define significant differences among groups. Also, Dunn's multiple comparisons test was used to compare differences of treated groups with control group. The data is shown as mean ± standard deviation and significant p values are marked by ****p<0.0001, ***p<0.001; **p<0.01; and *p<0.05.
Results :
5-FU treatment inhibited 4T1 tumor growth in vivo: The 4T1 tumor model was induced in syngeneic female Balb/C mice and low dose chemotherapy was initiated eight days post tumor inoculation for twelve days. Mice given doxorubicin at doses 2.5 mg/kg or 5 mg/kg showed a non-significant reduction of tumor growth compared to untreated group. However, 5-FU injection at dose of 50 mg/kg led to inhibit notably tumor growth of treated mice in comparison to the control mice (p=0.0139) (Figure 2).
5-FU depleted splenic and intertumoral MDSCs: On day 20 post tumor cell injection, mice were sacrifice d to evaluate effects of chemotherapeutic agents on frequency of MDSC population. Single cell suspensions of spleens and mammary tumors were obtained and analyzed by flow cytometry. MDSCs were phenotypically identified as double positive population (CD11b+/Gr-1+) which emitted both FITC and PerCP fluorescent. Twelve days after treatment, splenic MDSC decreased from 33.8% in the control group to 18.6 and 5.76% and also intratumoral MDSC reduced from 32.1 to 27.5% and 19.9 in 2.5 mg/kg and 5 mg/kg doxorubicin treated groups, respectively. So, doxorubicin at doses of 2.5 mg/kg and 5 mg/kg could reduce the MDSCs in both spleens and tumor microenvironments of 4T1 tumor bearing mice while only mice treated with dose of 5 mg/kg showed a significant decrease of splenic MDSCs comparing to control group (p=0.0382). Moreover, splenic MDSC in 5-FU (50 mg/kg) treated group decreased from 33.8% in the control group to 3.15% and intratumoral MDSC reduced from 32.1 to 9.53% in treated group. Thus, 5-FU treatment was associated with a significantly reduction in both splenic and interatumoral MDSCs comparing to control group (p=0.0276 and p=0.0067, respectively) (Figure 1).
Discussion :
Immune escape of tumor has been known as an important barrier against successful cancer immunotherapy 17,18. Inhibitory cells including Treg and MDSCs are the main components in formation of the tumor-induced immunosuppression and they accumulated in blood, spleen and tumor bed 19,20. MDSCs manipulation has become a main candidate for enhancing clinical efficacy of immunotherapy-based approaches and many strategies have been developed to target this immunosuppressive population 10. These strategies generally include induction of MDSCs differentiation to mature cells, prevention of their expansion and suppressive function, and depletion of MDSCs 21. Among these, anti-Gr-1 antibody can increase antitumor effects of ovalbumin vaccine and eradicate half of 3LL tumors in treated mice by MDSC inhibition 22. However, anti-Gr-1 antibody has the off-targeting effects on Gr-1+ mature granulocytes and can induce sever systemic immunosuppression in treated animals 23. Thus, agents with minor side effects selectively eliminating tumor-induced MDSCs achieve better outcomes in cancer immunotherapy. It has been reported a Fc-fusion protein (peptibody) specifically depletes MDSCs from multiple tumor models without adverse side effects on other immune cells 9. We focused on this peptibody in pervious study and showed peptibody treatment combing to an anti-HER2 monoclonal antibody for two weeks led to eradicate completely tumors in 60% of 4T1-HER2 tumor bearing mice 24. In addition to direct cytotoxic effects of conventional chemotherapeutic agents on tumor cells, these agents have been found to promote the antitumor immune responses in multiple murine tumor models 25. Immunologic effects of commonly anti-cancer agents can be explained by induction of DC maturation, reduction of Treg lymphocytes (such as cyclophosamide), MDSC depletion and promotion of immunologic cell death (such as doxorubicin) 26-28. Various studies have indicated that chemotherapeutic drugs such as 5-FU, doxorubicin, gemcitabine and paclitaxel are able to deplete MDSCs in tumor bearing hosts and improve anti-tumor efficacy of immune mediated treatments via restoration of anti-tumor immune responses 13,14,29. In this way, the aim in the present study was to evaluate effects of 5-FU and doxorubicin chemotherapeutic agents on MDSCs resident of spleen and tumor bed in 4T1 murine model. Our findings showed 5-FU treatment at 50 mg/kg for twelve days resulted in a significant reduction of MDSCs in both spleens and tumor beds from treated mice and inhibition of tumor growth in vivo.
5-FU, an antimetabolite agent, routinely administrates in multiple cancers including breast, colorectal, aerodigestive and pancreas cancer. It exerts cytotoxic effects on tumor cells through DNA/RNA damage and inhibition of thymidylate synthase 30. Not only 5-FU has direct cytotoxic effects on tumor cells but also it is able to activate immune responses in animal tumor models and even cancer patients 14. Immunomodulatory action of 5-FU is contributed to two main mechanisms: 1- reduction of tumors immunosuppressive burden by MDSC depletion 2- induction of immunologic cell death 31. Indeed, 5-FU treatment promotes antitumor immunity through reduction of MDSCs accumulation, production of danger alarmins and finally infiltration increase of immune cells into tumor beds (Figure 4). Combination of direct cytotoxic actions on tumor cells and recruitment of tumor evading immune cells to TME synergistically lead to develop an efficient antitumor response in 5-FU treated patients (Figure 4). In various studies, 5-FU could deplete MDSCs by inducing of apoptotic cell death and increase of infiltering cytotoxic CD8+ T lymphocytes 32. Three days post of 5-FU injection at a single dose (50 mg/kg), a significantly decrease of MDSCs from spleen (21.1-fold) and lung (8.1-fold) in 4T1 tumor model are found which it is sustained for five days after drug administration 15. Also, injection of multiple low dose (50 mg/kg) of 5-FU is associated with a remarkable decrease of both splenic and intratumoral MDSCs from B16 melanoma model and also induction of tumor specific immunity resulted in the prolonged survival and reduced tumor growth in treated mice 33. Furthermore, combination 5-FU with a DC vaccine improves anti-tumor efficacy of the cancer vaccine in F10/B16 melanoma model which shows additive effect of MDSC depletion in combinatory immunotherapies 34.
Other conventional chemotherapeutics such as cisplatin, gemcitabine, histone deacetylase inhibitors, and doxorubicin at low dose regimens also are able to decrease MDSCs frequency and subsequently augment T-dependent immune responses in 4T1 murine model 13,35-37. Histone deacetylase inhibitors, as chemical anti-cancer agents, can efficiently diminish MDSCs population present of blood, spleen and tumor bed post four weeks treatment and also increase perforin or INF-γproducing tumor infiltrating T cells in 4T1 mammary carcinoma model 38. In present study, a considerable decrease of splenic MDSCs frequency in 5 mg/kg dose of doxorubicin and a non-significant inhibition of 4T1 tumor growth in tread mice was observed. But, 5-FU was more effective in MDSCs depletion than doxorubicin and decreased number of both splenic and intratumoral MDSC to low levels following to notably inhibition of tumor growth in vivo. Doxorubicin treatment in 4T1 tumor bearing mice may associate with emergence of a doxorubicin-resistance subpopulation among tumor cells probably supports the MDSC expansion by the prostaglandin E2(PGE2) release 37. These resistance tumor cells may abrogate the doxorubicin effects on intratumoral MDSCs in 4T1 animal model. Furthermore, it has been suggested that MDSCs due to lower expression of thymidylate synthase are more sensitive to low doses of 5-FU agent and its higher concentration is needed to kill tumor cells comparing MDSCs 14. 5-FU mediated MDSC depletion could restore CTL responses involving of tumor elimination and subsequently provide on better control of tumor progression 31. Also, combinatory regiments including 5-FU used commonly in colorectal cancer could trigger immunologic death of tumor cells in which activated both innate and adaptive immune system 39. This action of 5-FU chemotherapy can be explained by release of DAMPs such as HMGB1, HSP70 from the chemically damaged tumor cells resulted in maturation induction of PRRs expressing APCs resident of tumor microenvironment 40.
On the other hand, superior effect of 5-FU on tumor-induced MDSCs might contribute to its myeloablative property which has the inhibitory effects on MDSCs expansion in highly aggressive 4T1 model with increased myelopoiesis 41-43. But exact mechanism of 5-FU action on MDSCs are largely unknown and more studies need to define 5-FU effects on MDSC biology induced in malignancies. Overall, 5-FU can consider as a potent anti-cancer agent to overcome on the tumor immune tolerance by specifically MDSCs elimination and to improve efficacy and outcomes of cancer immunotherapy settings.
Conclusion :
It was observed that 5-FU and doxorubicin had the cytotoxic effects on MDSCs in 4T1 tumor model and only 5-FU was able to inhibit significantly tumor growth in vivo by depletion of both splenic and intratumoral MDSCs. Data suggest that 5-FU may enhance the efficacy of anti-tumor treatments in cancer patients partly by inhibition of tumor-induced MDSCs.
Ethical approval :
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All animal experiments were performed according to guidelines of the Ethical Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1396.874).
Acknowledgement :
All animal experiments were performed according to guidelines of the Ethical Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1396.874).
Funding: This work was financially supported by Shahid Beheshti University of Medical Sciences (grant number: 12442), and Avicenna Research Institute (grant number: 96-017).
Conflict of Interest :
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
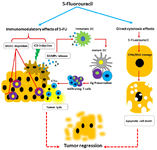
Figure 1. Anti-tumor effects of 5-Fluorouracil in chemotherapeutic regimens. 5-FU chemotherapeutic agent exerts anti-tumor actions by its effects of direct cytotoxic and also activation of anti-tumor immunity. 5-FU, as an antimetabolite agent, can inhibit DNA/ RNA synthesis by misincorporation and then upregulate expression of P53 and proapoptotic genes. Beyond of its direct effects on tumor cells, 5-FU is able to eliminate MDSCs resident tumor sites which it is associated to restore adoptive immune responses. Furthermore, immunomodulatory effect of 5-FU contributed to induce immunologic cell death (ICD) of chemically stressed tumor cells. ICD is featured by release of DAMPs such as HMGB1, HSP60 that trigger immune activation directed tumor antigens. Tumor evading CTLs along with apoptotic death of tumor cells lead to tumor regression in chemotherapy-based approaches. 5-FU: 5-Fluorouracil, MDSCs: Myeloid Derived Suppressor Cells, ICD: Immunologic Cell Death, DAMPs: Damage Associated Molecular Patterns, CTLs: Cytotoxic T Lymphocytes, DC: Dendritic Cell, Ag: Antigen.
|
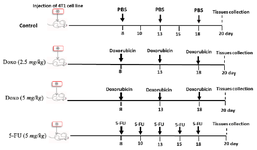
Figure 2. In vivo therapy plan of 4T1 bearing mice. Female Balb/C mice were orthotopically (mammary fat pad) challenged with 4T1 cell line on 0 day. Eight days post tumor inoculation, 4T1 tumor bearing mice were randomly divided into 4 groups (5 mice per group) received different treatments: PBS (control group), doxorubicin at doses of 2.5 mg/kg or 5 mg/kg and 5-FU at dose of 50 mg/kg for twelve days. Mice were sacrificed on day 20 post tumor cell injection and tissues were isolated to analyze by FACS.
|
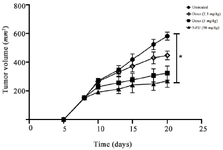
Figure 3. 5-FU agent significantly inhibited 4T1 tumor growth in vivo. 4T1 tumor challenged mice were treated with chemotherapeutic agents including doxorubicin or 5-FU drugs for twelve days. Mice given doxorubicin at doses 2.5 mg/kg or 5 mg/kg indicated a nonsignificant reduction of tumor volume compared to untreated group. But, 5-FU injection at dose of 50 mg/kg into tumor bearing mice resulted in markedly inhibition of tumor growth in comparison to the control mice (p=0.0139). The data is shown as mean ± standard deviation.
* p<0.05, n:5 mice per group.
|
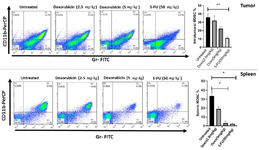
Figure 4. Depletion of splenic and intertumoral MDSCs by 5-FU and doxorubicin. Single cell suspensions were analyzed by flow cytometry to detect MDSCs from spleens and primary tumors. MDSCs express CD11b and Gr-1 surface markers and so gated in double positive population (FITC+/ PerCP+). Doxorubicin treated mice (dose of 5 mg/kg) showed a significant decrease in frequency of splenic MDSCs comparing untreated mice (p=0.0382). However, 5-FU treatment led to a notably depletion in both splenic and interatumoral MDSCs comparing to control group (p=0.0276 and p=0.0067, respectively). Plots show MDSCs frequency of one representative mouse from each group.
|
|