Overview on Immunopathology of Chronic Lymphocytic Leukemia and Tumor-Associated Antigens with Therapeutic Applications
-
 Shabani, Mahdi
Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran, Tel: +98 21 22439970; Fax: +98 21 22439970; E-mail: msshabani@sbmu.ac.ir
Shabani, Mahdi
Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran, Tel: +98 21 22439970; Fax: +98 21 22439970; E-mail: msshabani@sbmu.ac.ir
-
Rostamzadeh, Davoud
-
Medicinal Plants Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
-
Mansouri, Mansoure
-
Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Jeddi-Tehrani, Mahmood
-
Monoclonal Antibody Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR) , Tehran, Iran
Abstract: Chronic Lymphocytic Leukemia (CLL) is a clinically and biologically heterogeneous disease with a variable clinical course. The induction of a generalized state of immunosuppression, leading to susceptibility to infections and the failure of anti-tumor immune responses, is a key feature of the clinical course of CLL. In addition to B-cell receptor (BCR) signaling in CLL, several receptor tyrosine kinases (RTKs) have been reported to be constitutively active in leukemic B cells, resulting in promoted survival and resistance to apoptosis induced by chemotherapy. Several treatment options are available for CLL, including a watch-and-wait strategy, chemotherapy, targeted therapies, immunotherapies such as adoptive cellular therapy (CAR T-Cell Therapy), stem cell transplantation (allogeneic transplantation), radiation therapy and surgery. The identification of Tumor-Associated Antigens (TAAs) is the bottleneck of tumor immunology and immunotherapy, serving as promising targets for precise diagnosis, monitoring, or therapeutic approaches. Numerous TAAs have been identified, and their application in immunotherapy holds promise for the treatment of CLL. Furthermore, extensive ongoing research aims to identify new cancer TAAs. In this review, our objective is to provide a comprehensive overview of CLL immunology and recent findings regarding advances in TAAs with therapeutic applications in CLL.
Introduction :
Chronic Lymphocytic Leukemia (CLL), a member of the Non-Hodgkin Lymphoma (NHL) family, is a common lymphoproliferative disorder with high heterogeneity in clinical behavior that accounts for approximately up to 30% of all adult leukemias 1. The median age at CLL diagnosis is around 67-72 years 2. Its diagnosis is based on absolute number (more than 5000 B-lymphocytes/µl for the duration of at least 3 months) and clonal proliferation of B cells, that represent a specific immune-phenotype (CD19+, CD20+, and CD23+) that is frequently associated with expression of CD5 antigen (95% of patients) within the peripheral blood, bone marrow as well as other lymphoid organs 3,4. Clonal proliferation of circulating B cells must be confirmed by flow cytometry 2. Marrow aspirate and biopsy is not mandatory for diagnosis of CLL, but it is recommended to differentiate between autoimmune cytopenias (anemia, and thrombocytopenia), which is not associated with leukemia-cell infiltration of the normal bone marrow 5. Marrow biopsy is highly recommended to start treatment in a clinical trial with potentially myelosuppressive agents 5. Lymph node biopsy is not necessary for diagnosis of CLL but is only recommended for diagnosis of transformation into a more aggressive type of lymphoma (suspected Richter’s transformation) 5,6.
Standard therapies in CLL have emerged from the use of alkylating agents and then switched toward more aggressive immunotherapy-based regimens in order to improve response rates and extended survival 7. For several decades, administration of chlorambucil has been considered as the "gold standard" first-line therapeutic approach in CLL 7. Over the recent years, significant progress has been made in the treatment of CLL and several new drugs have been approved such as cytostatic agents (purine analogs and bendamustine), monoclonal Antibodies (mAb) such as rituximab, ofatumumab, obinutuzumab, alemtuzumab, agents targeting B-cell receptor signaling (idelalisib, ibrutinib and acalabrutinib), BCL-2 inhibitors (venetoclax), BTK inhibitor (zanubrutinib and ibrutinib) and immunomodulatory drugs 2,8-10. Constitutive activation of several tyrosine kinases in the form of receptors and non-receptors involved in B cell survival and resistance to apoptosis have been identified in CLL 11,12. Even with introduction of these drugs, identification of new ideal Tumor-Associated Antigens (TAAs) is the bottleneck of tumor immunotherapy approaches. Numerous TAAs have been identified and their application in immunotherapy is promising for treatment of CLL. Besides, research for identification of new cancer TAAs has been ongoing most extensively. In this review, aim is to give a comprehensive overview of CLL immunology and recent findings regarding the advances in TAAs and their therapeutic applications in CLL.
Immunological features of CLL patients: Alterations of B and T lymphocytes: A key feature of the clinical course of CLL is immunosuppression, causing augmented susceptibility to infections and failure of anti-tumor immune responses 13. CLL patients show abnormal distribution pattern of circulating T cell subpopulations in peripheral blood. A surprising finding related to the immune cells in CLL was the increase in absolute number of circulating CD8+ T cells 14. These CD8+ T cells secrete high levels of IL-4 15. The IL-4 producing CD8+ T cells displayed increased expression of CD30. It has been revealed that ligation of CD30L on the surface of CLL B-cells stimulates their production of TNF-α and enhances their proliferation 16. Furthermore, IL-4 is able to prevent apoptosis of B-CLL cells in a BCL-2 dependent manner and therefore plays an important role in pathogenesis of CLL disease 17. Leukemic CLL cells induce CD30 and downregulate CD40L expression on T cells in OX40L and IL-4 dependent as well as in contact-dependent manners. Contrary to malignant B cells, upregulated CD30+ T cells inhibit CD40L mediated immunoglobulin class switching by engaging CD30L in non-malignant B cells 18. Leukemic CLL cells are prominent in secreting several cytokines. In this context, CLL cells contribute to the T cell defects via secretion of IL-6. IL-9 secreted by leukemic cells negatively modulates the cytotoxic T cell-mediated killing by inducing PD-1 expression 19. Stimulation of healthy T cells in the presence of tumor derived supernatant containing high levels of IL-6 increases their production of IL-4, and causes them to show impaired upregulation of CD40L expression 20. Furthermore, elevated level of IL-6 in CLL patients is correlated with poor survival and diverse disease features 21. Additionally, disrupted B cell function in CLL patients has been reported 22.
Global gene expression profiles of peripheral blood T cells from CLL patients revealed altered gene expression profiles compared to healthy donors 23. Gene analysis demonstrated expression of genes mostly involved in cell differentiation, proliferation, survival, cytoskeleton formation, and vesicle trafficking of CD4+ T cells and cytoskeleton formation, intracellular transportation, vesicle trafficking, or cellular secretion as well as cytotoxicity pathways in CD8+ T cells. Alterations in cytoskeletal relevant gene expression resulting in functional defects in actin polymerization, and consequently CLL T cells exhibit defects in formation of immunological synapse with antigen presenting cells (APCs) 23.
Frequency of regulatory T (Treg) cells: Abnormally high regulatory T (Treg) cells absolute count observed in CLL patients is considered a critical mechanism of immunosuppression in these patients. CLL patients unusually show increased frequencies of CD4+CD25hiFOXP3+ Tregs which may be correlated with the disease status such as tumor progression and expansion as well as clinically advanced disease 24,25. In addition, raised numbers of Tregs in CLL patients is correlated with decreased T cell responses against viral and tumor antigens. Treg cells from CLL patients displayed reduced amounts of CD25 expression intensity and may inhibit anti-tumor T cell responses by releasing soluble CD25 resulting in inhibition of Th1 differentiation 26. Besides immunosuppressive effects of Tregs, CD4+ T cells may be essential in controlling immune related diseases. Both CD4+ FoxP3+ and CD4+ FoxP3− T cells have been demonstrated to act as cytotoxic populations of CD4+ T cells and express cytolytic markers like Fas ligand and CD107a, rendering them to kill autologous leukemic B cells in vitro 27. Increased frequency of Treg cells in CLL patients may be due to significantly elevated levels of anti-apoptotic BCL-2 resulting in decreased susceptibility to apoptosis and induction of Treg formation through CD27-CD70 co-stimulation in the Lymph Node (LN) follicular proliferation centers 27. TIGIT (T cell immunoreceptor with Ig and ITIM domains) expressing CD4+ T cells are enriched in CLL and these cells provide a supportive microenvironment for CLL cells, representing a potential therapeutic target for CLL treatment 28. In addition, it has been elucidated that CLL T cells show increased expression of CD57, CD71, CD69, and HLA-DR and decreased expression of CD28 and CD62L, which represent their systemic and chronically activated phenotypes 29.
Abnormality in NK cells: Impaired Natural Killer (NK) cell activity has also been reported in CLL patients. NK cells from CLL patients showed lack of cytoplasmic azurophilic granules resulting in reduced ability to lyse leukemia cell lines 30. IL-2 is able to restore the impaired NK cell activity and increased granularity of the Large Granular Lymphocyte (LGL) subset 31. However, based on downregulation of NK cell function by CLL cells, it has been suggested that malignant CLL cells may be capable of secreting immunosuppressive factors that down-regulate T cell and NK functions 32. Similar to CLL T cells, NK cells from CLL patients show defective actin polymerization and impaired immunological synapse formation which affect the NK-cell mediated cytotoxic mechanisms 33. CD3+CD16+CD56+ NKT cell frequencies appear to be of clinical significance, as a decrease in number of these cells is related to disease progression and higher risk of death in CLL patients 34. Therefore, development of a chimeric antigen-receptor (CAR)-NK therapy strategy against the CLL cells such as CD19-specific CAR-NK cells have given great interest for treatment of hematological malignancies, particularly CLL 35,36.
In addition, dysregulation of monocyte and neutrophil functions has been reported in CLL. CLL monocytes and neutrophils have been shown to be deficient in myeloperoxidase and lysozyme activities. These events may affect CLL B-cell survival through changes in the secretion of TNF superfamily proteins 37.
Genomic alteration of CLL cells: Recently, comprehensive description of putative genomic landscape of CLL by Whole Exome Sequencing (WES) in large cohorts revealed that loss or addition of large chromosomal material (e.g., 13q, 11q, 17p deletions and trisomy 12) may conceivably be involved in disease initiation, aggressiveness and progression 38. The most frequent cytogenetic abnormality in the CLL leukemic cells is deletion on the long arm of chromosome 13q14 [del(13q14) that occurs in more than half of all cases (%55)] 2,39. Deletion at 13q14 is also associated with most mantle cell lymphomas (%50), multiple myeloma (%16-40) and prostate cancers (%60) and is related to their pathogenesis 40. miR15 and miR16 were recently identified to be exactly located on chromosome 13q14 and both miRNAs are deleted or downregulated in most CLL sample cases (≈68%) 39. Genetically modified mice carrying a targeted deletion of the DLEU2/miR-15a/16-1 cluster recapitulates many features of CLL including development of CLL, monoclonal B cell lymphocytosis-like disorder, and lymphoma 41. These data have suggested a crucial role for DLEU2/miR-15a/16-1 locus in controlling the expansion for the mature B cell pool and may harbor unknown tumor suppressor genes in B cell lineage and indicate that DLEU2/miR-15a/16-1 locus may play a critical and direct role in CLL leukemogenesis and pathogenesis 41.
Chromosome region 11q22.3–q23.1 frequently harbors the Ataxia Telangiectasia Mutated (ATM) gene which activates p53 protein in response to DNA Double-Strand Breaks (DSBs) 42. Prevalence of ATM mutations have been reported in 12% of CLL patients and approximately in one-third of CLL patients harboring 11q23 deletion 42. Deletions of ATM (11q22–q23) have been found in approximately 25% of patients with more advanced clinical stages of CLL and have been rarely found in early stage disease (10%) 43. Patients harboring mutations in 11q23 exhibit more rapid disease progression, extensive LN involvement and reduced overall survival 44. Trisomy 12 is one of the most frequent aberrations (10-20%) with unidentified susceptibility genes involved in the pathogenesis of CLL 43. However, in line with these observations, further studies remain to be performed to explore the probable correlation between the incidence of trisomy 12 and relevance of CLL prognosis.
Another chromosomal aberration is deletion in band 17p13 (involving the p53 locus) which is found in 5-8% of CLL patients at diagnosis or in chemotherapy-naïve CLL patients 45,46. The association between poor prognosis, drug resistance and poor survival of CLL patients with 17p deletion or p53 mutation has been reported in several studies 47-51. In patients diagnosed with 17p deletion, most cases have shown mutations in the remaining TP53 allele (80%). Furthermore, in cases without deletion in 17p, TP53 mutation is rare, but has a similar impact on chemotherapy response and overall survival 52.
Using WES to characterize the genetic landscape of CLL patients, 44 recurrently mutated genes and 11 recurrent somatic copy number variations were identified 53. These mainly include the genes NOTCH1, MYD88, KLHL6, TP53, ATM, SF3B1, FBXW7, POT1, CHD2, RPS15, IKZF3, ZNF292, PAX5, ZMYM3, ARID1A, XPO1, and PTPN11 53-55. These studies provide a comprehensive catalog of somatic mutations of the CLL genomic landscape which provides useful results in targeting several well-known genetic pathways and treatment of CLL and other malignancies.
Signaling pathways in CLL cells involved in pathogenesis and disease progression: Ligand-dependent B-cell receptor signaling pathways in B-CLL: Activation via B-Cell Receptor (BCR) signaling can trigger several cascades of signaling events that lead to multiple events in normal and malignant B cells including B cell selection, proliferation, differentiation, antibody production and isotype switching 56. Induction of BCR signaling pathway either via antigen (ligand-dependent) or "tonic signaling" (ligand-independent) seems to play an essential pro-survival role for the survival, growth and pathogenesis of CLL cells and in other NHLs 57. Analysis of Gene Expression Profiling (GEP) within the tissue microenvironment of lymphatic tissues explored BCR signaling as the most predominant signaling pathway engaged in CLL which promoted maintenance, proliferation and survival of CLL cells in vivo 58. Among genes upregulated in LN resident CLL cells, BCR signaling shows a highly overrepresented profile in cooperation with upregulation of c-MYC and NF-κB, suggesting the LN as an important site in CLL pathogenesis 58. Furthermore, genes involved in BCR signaling are stronger in clinically more aggressive CLL which supports the notion that inhibition of BCR signaling is an appropriate therapeutic strategy in CLL 58.
Growing evidence supports the idea that recognition of various autoantigens and other environmental or microbial antigens by polyreactive/autoreactive BCRs from U-CLL patients may lead CLL cells to programmed secretion of multispecific autoantibodies. It has been shown that CLL BCRs could bind to non-muscle Myosin Heavy chain IIA (MYHIIA) which probably stimulates CLL cells toward development, survival, and expansion 59. CLL cells have also been reported to be autoreactive against Fc domains of IgG, histones, cardiolipins, cytoskeletal components 60, ssDNA, dsDNA 61, apoptotic cells, oxidized Low-Density Lipoprotein [oxLDL], 62 and some pathogenic bacteria and fungi 62,63. CLL B cells, irrespective of their V gene mutational status, represent features of activated and of antigen-experienced B cells based on the overexpression of the activation markers CD23, CD25, CD69, and CD71 61.
Ligand-independent B-cell receptor signaling pathways in CLL: It is well known that tonic signaling of BCR is essential for development, maintenance, and survival of normal B cells 64. It has been shown that unmanipulated primary or freshly isolated CLL cells display elevated intrinsic tonic signaling activity 65. Src kinase Lyn, plays an essential role in transducing survival or apoptosis signaling cascade which is triggered following BCR engagement 66. Analysis of protein tyrosine phosphorylation downstream of BCR in normal and CLL cells revealed overexpression and significant constitutive activation of Lyn in freshly isolated leukemic cells 67. Mice deficient in expression of Src kinases Lyn, Fyn, and Blk showed increased levels of apoptosis in pre-B cells 68. Syk as a key protein kinase downstream of BCR has been shown to be constitutively phosphorylated and active on the activating Y352 residue in CLL B cells and Syk inhibitor induced leukemic cell apoptosis 69. Constitutive activation of Syk has been shown in several common B-cell malignancies indicating a role for antigen-independent activation of Syk in the pathogenesis of these diseases 70,71. Therefore, Syk can be a potential target for inducing apoptosis in CLL leukemic cells and an appropriate therapeutic target by disrupting antigen-dependent and independent signaling pathways in CLL. Furthermore, CLL cells have been shown to exhibit constitutive phosphorylation of Erk, NFAT and subunits of NF-κB 72,73. This constitutive activation of tonic BCR signaling suggests that antigen-independent BCR signaling might be associated with oncogenic mechanisms and clinical relevance in CLL and induces resistance of the leukemic B-cells to therapy. In particular, unmanipulated CLL cells show different N-glycosylation patterns in the μ-constant region in IgM including glycoform similar to normal B cells and immature mannosylation. In contrast to IgVH mutated CLL (M-CLL) cells, unmutated CLL (U-CLL) cells display elevated levels of mannosylated surface μ chains which may increase the ongoing interaction with local lectin-bearing stromal cells 74. Immunophenotypic analysis of leukemic cells from CLL patients have shown characteristics of activated and antigen-experienced B cells irrespective of their V gene mutational status 61.
Growing evidence supports the idea that recognition of various autoantigens and other environmental or microbial antigens by polyreactive/autoreactive BCRs from U-CLL patients may lead CLL cells to programmed secretion of multispecific autoantibodies. It has been shown that CLL BCRs could bind to non-muscle myosin heavy chain IIA (MYHIIA) which probably stimulates CLL cells toward development, survival, and expansion 59. CLL cells have also been reported to be autoreactive against Fc domains of IgG, histones, cardiolipins, cytoskeletal components 60, ssDNA, dsDNA 61, apoptotic cells, oxLDL, 62 and some pathogenic bacteria and fungi 62,63. As mentioned above, CLL B cells, irrespective of their V gene mutational status, represent features of activated and of antigen-experienced B cells based on the overexpression of the activation markers CD23, CD25, CD69, and CD71 61.
Non-receptor tyrosine kinases signaling in CLL B cells: In addition to BCR signaling in CLL, several Receptor Tyrosine Kinases (RTKs) have been reported to be constitutively active in leukemic cells resulting in promoted survival and resistance to apoptosis induced by chemotherapeutic drugs. RTKs are cell surface glycoproteins and a subclass of tyrosine kinases involved in the regulation of various cellular processes, such as proliferation, carcinogenesis, growth, differentiation, survival, signaling and migration 75. For example, Insulin-like Growth Factor-I (IGF-I) released by stromal cell in bone marrow plays an important role in regulating B lymphopoiesis by enhancing the differentiation and development of normal pro-B to pre-B lymphocytes as well as stimulating μ-heavy chain gene rearrangement and protein expression 76. For the first time, IGF-I has been reported to be expressed by 44% of CLL patients and its expression has been positively correlated with BCL-2 anti-apoptotic protein expression and CLL cells survival 77, which supports the notion that the paracrine/autocrine control of CLL cells occurs through interaction of IGF-1 and IGF-1R. High level expression of Insulin Receptor (INSR) has been reported in the majority of CLL cases with 11q-del as compared to CLL cases with other genomic abnormalities and normal B cells in these patients 78, while normal B cells from peripheral blood express moderate levels of INSR 78. Interestingly, higher expression of INSR has been shown to be correlated with shorter time to first therapy and shorter overall survival 78. These results highlighted the IGF-1 and INSR as a potential therapeutic target in CLL.
Receptor tyrosine kinase-like Orphan Receptor (ROR) protein is a member of RTKs that play an important role in developmental processes including skeletal and neuronal development, cell movement and cell polarity 79. ROR1 has been shown to be highly expressed in CLL and several other malignancies with functional activity and its involvement in tumor cells signaling 80-85. Gene expression profiling of CLL B cells showed increased expression of ROR1 in leukemic cells 77,86. The majority of CLL cells (70% mean), but not normal B cells, and other normal blood cells, exhibited ROR1 surface expression and mRNA levels uniformly 87,88. No correlation between ROR1 protein expression and IgVH mutated and unmutated cases as well as progressive and non-progressive CLL patients has been reported 87-90. Unique surface expression of ROR1 by CLL B cells, but not normal B cells and other tissues merits further studies of its role in the pathobiology of CLL and makes it a potential therapeutic target for CLL 84. Currently a mAb specific to ROR1 (cirmtuzumab) has been tested in phase I clinical trial (NCT02222688) 91 and a few Small Molecule Inhibitors (SMI) are in preclinical stages for targeting ROR1 80,92.
In CLL, Vascular Endothelial Growth Factor A (VEGFA) promotes CLL B cell survival and progression which is most likely to be associated to activating the STAT1/STAT3 signaling pathway and upregulating the critical anti-apoptotic protein Myeloid cell leukemia-1 (Mcl-1) in leukemic B cells 93. Since overexpression of VEGF and Mcl-1 are associated with poor prognosis of B CLL, inhibition of VEGF and related signaling pathways can be therapeutically targeted for treatment of CLL. Furthermore, it has been proven that leukemic B cells spontaneously secrete both pro- and anti-angiogenic molecules, including basic Fibroblast Growth Factors (bFGF), VEGF and T thrombospondin-1 (TSP-1). Because of the co-expression of angiogenic molecules and receptors, CLL B cell biology is directly affected by autocrine pathways of stimulation 94. In addition, micro vessel density of bone marrow in CLL patients is correlated positively with the clinical stage of the disease 95. Some studies have also reported increased serum levels of VEGF and bFGF in CLL that can be considered useful targets for predicting the risk of disease progression 96.
Axl is another important RTK involved in pro-survival signaling pathway in CLL. Axl alongside with Tyro3 and Mer are members of the TAM receptor tyrosine kinase family 97. Axl was primarily identified in patients with Chronic Myelogenous Leukemia (CML) 98. Axl activation and signaling have been involved in several cellular responses, including cell survival, proliferation, migration, phagocytosis, adhesion and angiogenesis as well as implication in multiple types of human cancer, inflammatory, autoimmune, development of atherosclerosis and kidney diseases 99. Analysis of freshly isolated CLL B cells has constitutively shown activation of Axl which is correlated with other constitutively phosphorylated kinases, including Lyn, phosphoinositide-3 kinase, SyK/ζ-associated 70 kDa protein, phospholipase Cγ2 in CLL cells 100. Inhibition of Src/Abl kinase or specific-inhibition of Axl may induce massive CLL B-cell apoptosis 100 which can be an attractive target for treatment of CLL.
c-MET is another member of RTKs that acts as a high affinity receptor for Hepatocyte Growth Factor (HGF)/scatter factor 101. The HGF/c-MET signaling pathway has been reported to be dysregulated in a wide range of human malignancies with poor clinical outcomes and drug resistance 102. Furthermore, it has been demonstrated that aberrant expression of c-MET and HGF/c-met pathways is in favor of survival and apoptotic resistance in CLL, but not on normal donor CD19+ cells. Increased expression of c-MET has also been associated with decreased expression of adhesion molecules 103.
These results suggest that RTKs inhibition can be potential pharmacological targets in CLL and recommend that combining anti-RTK agents with more traditional therapeutic drugs may emerge as new oncological targets for antibody-based therapy.
Targeted therapy drugs for chronic lymphocytic leukemia: There are several treatment options for CLL including chemotherapy, radiotherapy, surgery, stem cell transplantation (allogeneic transplantation), target based therapies such as mAbs 104, SMI (ibrutinib, acalabrutinib, and zanubrutinib) 105,106 and immunotherapy methods like adoptive cellular therapy (CAR T-cell therapy) 107. There are a number of targets to specifically target malignant B cells, such as CD20, CD19, CD52, CD70, CD74, CD40, and CD37 (Figure 1, Table 1) 107,108. In addition, there are also numerous mAbs in clinical development for CLL therapy (Table 1) 107,108.
CD20; a pan B cell surface glycoprotein: CD20 is a potential cell surface target for elimination of circulating CD20-expressing B cells such as B cell malignancies. CD20 is a hydrophobic glycosylated transmembrane protein and its expression is relatively limited to the cell surface of normal mature B, CLL B cells and on >90% of B cell NHL, but not stem cells, pro-B cells and plasma cells 109. However, the precise function of CD20 remains poorly understood. CD20 knockout mice have been shown to exhibit normal B cell development but are deficient in CD19-induced calcium responses and BCR signaling 110. Since internalization and shedding of specific targets may potentially determine the efficacy of targeted therapy, CD20 appears neither undergo cell-surface shedding nor internalization 111. In addition, its specific expression on B cells makes it a specific marker for targeted therapy in CLL. Rituximab is a chimeric anti-human CD20 IgG1 κ murine/human immunoglobulin containing both murine light- and heavy-chain variable region sequences with human constant region sequences (Figure 1) 108. It has been demonstrated that rituximab exerts its cytotoxic effect mainly by Antibody Dependent Cellular Cytotoxicity (ADCC), Complement Dependent Cytotoxicity (CDC), Antibody Dependent Phagocytosis (ADP and direct apoptosis with a cross-linking antibody 107,108. Since CLL cells express relatively low levels of CD20, these cells show little susceptibility to CDC mediated by rituximab 112. However, CLL cells have been demonstrated to be susceptible to CD20-shedding after treatment and reduce the ability of rituximab to induce CDC 113. Monocyte mediated ADP and NK cell mediated ADCC have also been reported in CLL cells exposed to rituximab in vitro 114. It has been shown that Treg cells strikingly diminish NK cell mediated ADCC function toward rituximab-labeled tumor cells 115.
A significant number of patients have been observed to relapse following treatment with rituximab. Therefore, development of novel anti-CD20 mAbs with improved therapeutic efficacy is necessary. A second-generation of fully humanized anti-CD20, ofatumumab recognizes a unique and different epitope on CD20 than rituximab, has been developed. This mAb exerts similar ADCC, potent CDC, slower off-rate, more stable CD20 binding and direct apoptosis after cross-linking to rituximab 108,116. Type II glycoengineered fully humanized high affinity mAb to CD20 epitope, GA101/Obinutuzumab showed greater effect to induce ADCC and higher capacity to deplete CLL B cells from peripheral blood compared to rituximab 117-119. Obinutuzumab was approved by the Food and Drug Administration (FDA) in November 2013 for use in combination with chlorambucil for the treatment of patients with previously untreated CLL 120. Clinical trial of the triple combination of obinutuzumab, ibrutinib, and venetoclax (GIVe regimen) presented a promising outcome for this combination regimen and thus introduced it as a first-line treatment for patients with high-risk CLL 121.
CD52; a small immunomodulator glycoprotein: Alemtuzumab (Campath-1H) is a fully humanized IgG1 κ mAb directed against cell-surface antigen CD52 122. CD52 is a 21-28 kDa glycosylphosphatidyl-inosltol (GPI)-linked glycoprotein expressed at high levels on all B and T cells. It is expressed at lower levels on monocytes and macrophages, eosinophils, NK cells, and monocyte-derived dendritic cells 123. Overexpression of CD52 antigen has also been demonstrated on T cell Prolymphocytic Leukemia (T-PLL) and CLL 124. Immunologically, alemtuzumab can mediate CDC, ADCC by virtue of its IgG Fc region and can induce direct caspase-independent cell death through a membrane raft-dependent mechanism (Figure 1) 108,125. Clinical studies have shown that alemtuzumab could be an effective therapy for CLL patients with high-risk cytogenetic markers including p53 mutations or 17p and 11q deletions as well as other genomic aberrations 126,127. Therefore, alemtuzumab might be a reasonable treatment for CLL patients with these poor prognostic features. Although alemtuzumab has been approved for the treatment of CLL, due to the strategic decision of Sanofi company, the license of this drug was withdrawn in August/September 2012 from the clinic and it is only available through an international compassionate use program 2.
CD19; a B-cell surface glycoprotein: Co-stimulatory molecule CD19, is a transmembrane glycoprotein of immunoglobulin superfamily and is a B cell marker that is highly expressed on most malignant B cells, especially in leukemic CLL cells and is considered as a potential target for immunotherapies 128. Recent advances in engineered antibody technology have led to development of a series of modified antibodies for targeting CD19. MDX-1342 (NCT00593944) and MEDI-551 (NCT01466153) are non-fucosylated fully human mAbs specifically directed against CD19 that acts predominantly via ADCC and ADP (Figure 1). Data from a phase 2 clinical trial, has shown that MEDI-551 has demonstrated a 30% response rate in relapsed/refractory (RR) CLL as a monotherapy and further study showed clinical activity and comparable safety compared to rituximab and bendamustine (NCT01466153) 129. XmAb5574 (MOR00208) is a new Fc-engineered anti CD19 mAb with an engineered Fc region to enhance Fcγ receptor binding affinity for improving ADCC and ADP 130. Phase 1 clinical trial as a monotherapy (NCT01161511) exhibited safety and preliminary efficacy in RR CLL patients 130. Phase 2 XmAb5574 in combination with lenalidomide for RR CLL patients is under investigation (NCT02005289).
CD37; a heavily glycosylated glycoprotein: Another lineage-specific B cell target, CD37, is a heavily glycosylated 40 to 52 kDa glycoprotein member of the transmembrane 4 superfamily (TM4SF) of tetraspanin family of proteins and is considered to be an attractive therapeutic target for CLL targeted therapy 131. Otlertuzumab (TRU-016) is a novel humanized anti-CD37 mAb that shows potential capacity to induce ADCC and caspase-independent cell death against CLL cells (Figure 1) 131. Otlertuzumab includes anti-CD37 antibody variable regions linked to immunoglobulin constant domains and is categorized as a small modular immunopharmaceutical (SMIP). Phase 1 escalation evaluation of otlertuzumab in RR CLL patients has demonstrated 23% response rate with acceptable toxicity 132.
CD40; a TNF receptor superfamily member: A member of the Tumour Necrosis Factor Receptor (TNFR) superfamily, CD40, is highly expressed by normal and neoplastic CLL cells 133. CD40 activation has been found to be involved in enhanced cytokine secretion, proliferation and survival of neoplastic B cells, triggering phosphorylation of downstream signaling molecules associated to oncogenic mechanisms and clinical relevance in CLL. It induces resistance of the leukemic B cells to therapy such as ERK 1/2 and upregulates Mcl-1 and Bcl-xl 134. Humanized anti-CD40 antagonist antibody, lucatumumab (HCD122), blocks the interaction of CD40 with CD40L and inhibits CD40L-induced activation of signaling pathways, survival, cytokine secretion as well as mediating ADCC (Figure 1) 135. Data from a phase I study of lucatumumab has shown that 17 out of 27 CLL patients have stable disease and acceptable toxicity 136. Another anti-CD40 mAb, dacetuzumab (SGN40) mediates ADCC against CLL cells and its effect is further enhanced in combination with lenalidomide 136. However, phase I dose escalation study as a single agent demonstrated minimal clinical activity (NCT00283101) 137. Further studies are proposed to be performed on dacetuzumab in combination with other chronic lymphocytic leukemia therapies.
Tumor associated antigens: potential targets for CLL immunotherapy: Identification of human Tumor associated antigens (TAAs) is one of the main goals of targeted therapy and tumor immunology that serve as promising targets for diagnosis, disease monitoring and therapeutic approaches. Several investigations have been performed to detect new TAAs (Table 2). TAAs mostly are the mutational status of normal proteins expressed by normal tissues, aberrantly expressed normal genes, or genes encoding viral proteins which make them potential targets for immunotherapeutic vaccines 138. These antigens should contain peptide sequences that are recognized by MHC molecules. Recently, several TAAs with the potential to be specifically recognized by T cells and induce a specific Cytotoxic T Cell (CTL) response and specific CTL-mediated antitumor immune response have been characterized in CLL 139,140.
CD23; a low-affinity receptor for IgE (Fc3RII): CD23 that is expressed on the surface of B cells, belongs to type II integral membrane proteins (C-type lectin family) 141. CD23 is constitutively and strongly overexpressed by leukemic B cells with significant prognostic importance in CLL 142. Serum CD23 level has been shown to be selectively elevated in CLL patients and significantly contributes to significantly worsening the prognosis and overall survival 143. Evaluation of serum level of CD23 in the early stage (stage A) of the disease can provide significant prognostic information, but during the course of the disease it is presumed to help to the early detection of patients who will rapidly progress to upper stages 143. Moreover, analysis of GEF has revealed 5.9-fold increase in expression of CD23 in leukemic B cells compared to normal B cells 144. Previous studies have demonstrated that CD23 could be naturally processed and presented in the context of MHC molecules. They showed that HLA-A2-restricted specific CTLs for CD23-derived peptides from CLL patients efficiently recognized naive and CD40L-activated autologous malignant CLL cells 145. These findings strongly support the notion that CD23 can potentially serve as a specific TAA in CLL and may also be employed as an attractive therapeutic target for the development of T cell–based immunotherapeutic approaches such as clinical vaccination trials or adoptive T cell transfer of B-CLL and other CD23+ B-cell malignancies. phase 1, dose escalation and schedule optimization study of the macaque-human primatized mAb (lumiliximab), directed against CD23 in RR CLL patients has shown minimal activity, acceptable toxicity and well-tolerated profile (NCT00046488) 146. Although, phase 1/2 study of lumiliximab combined with fludarabine, cyclophosphamide, and rituximab (FCR) in patients with RR CLL shows potential benefit (NCT00103558) 147, phase 3 did not confirm the ability of lumiliximab to improve response to treatment and Progression-Free Survival (PFS) when FCR plus lumiliximab was compared with FCR alone 108.
MDM2; the human homolog of murine double-minute 2 oncoprotein: MDM2, also known as MDM2 E3 ubiquitin ligase, is typically expressed in the nucleus, which translocates to the nucleus following its activation and acts as an effective tumor suppressor protein p53 negative regulator 148. Upon activation, MDM2 binds to the amino-terminus of P53 and targets it for ubiquitinylation and subsequent proteasomal degradation and therefore exerts its oncogenic affect by inhibiting its transcriptional activity 148. MDM2 protein overexpression has been reported in multiple types of human cancers, especially in tumors with a wild-type p53, such as breast cancer, colorectal cancer, glioblastoma, cutaneous melanoma, differentiated liposarcoma, CLL, NHL, Hodgkin lymphomas, and osteosarcoma 149-152. Northern blot analysis showed 10-fold higher expression of MDM2 RNA levels in neoplastic B cells from B-CLL or NHL patients than in normal B cells 152. In addition, MDM2 gene overexpression has been reported to be more frequent in patients at advanced clinical stages (stage IV) than in those at lower clinical stages (stage II or III) 152. These results may suggest an important role for MDM2 in tumorigenicity and/or disease progression of CLL. Mayr et al. defined MDM2 as a novel TAA recognized by CD8+ autologous T cells in B-CLL and also showed that MDM2 expression was detectable in 85% of CLL patients. They identified MDM2 HLA-A2-restricted specific CTLs that recognized T2 target cells loaded with MDM2 peptides and autologous MDM2 overexpressing CLL cells which naturally processed and presented it in the context of MHC-I molecules 153. Inhibition of the MDM2-p53 protein-protein interaction or MDM2 E3 ligase function promotes steady state p53 levels, inhibits its p53-degradation activity and in some cancer cells stimulates apoptosis 154,155. Investigation of apoptosis induction after treatment of highly pure CLL tumor cells ex vivo with 2 MDM2 inhibitors, Nutlin-3 and MI-63 showed that p53 status potentially determines the CLL cells response to MDM2 inhibitors 155. These results indicated that MDM2 could be an interesting TAA target in therapeutic approaches in CLL patients. Furthermore, in the setting of CLL, utilizing MDM2 inhibitors in combination with drugs less sensitive to p53 mutation status, like alemtuzumab or flavopiridol (a potent CDK4-blocking activity), might be more effective immunotherapeutic strategies in treatment of CLL. The results of phase I clinical study of RG7112 (NCT00623870), a small-molecule MDM2 antagonist showed its clinical activity in RR AML and CLL patients 156.
Survivin: Survivin, a member of inhibitor of apoptosis protein family (IAPs) seems to play a key role in suppression of apoptosis and regulation of cell division 157. Overexpression of survivin has been reported in several cancer types, including bladder, lung, breast, stomach, oesophagus, liver, and ovarian cancers, ALL and AML 158. The overexpression of survivin in most human cancers suggests its important role in tumor progression. Aberrant expression of survivin is a biomarker of poor prognosis and in drug/radiation resistance 159. Survivin overexpression has been found to happen in Bone Marrow (BM) and LNs, especially in pseudofollicles from patients with B-CLL, as well as CD40 stimulated B-CLL cells 160. Survivin expressing cells actively proliferated and uniformly and intensely expressed BCL-2, indicating a remarkable resistance to apoptosis 160. A vaccine targeting survivin could potentially target a proliferation compartment of B-CLL. The immune reaction takes place in pseudofollicles of LNs and provides a new way of eliminating B-CLL by promoting the eradication of the proliferating tumor cell pool 160. Nonetheless, survivin-reactive T cells have been detected in the peripheral blood of patients with CLL. Specific CTL responses against two survivin-derived peptide epitopes were also identified in CLL patients, but not in healthy controls 161. Effective and specific in-vitro CTL responses against survivin have also been induced by autologous Dendritic Cells (DCs) pulsed with soluble recombinant survivin protein. These survivin-specific CTLs were capable of recognizing Epstein-Barr virus (EBV) B lymphocytes transfected with survivin cDNA or allogeneic lung tumor cells 162. In other studies, induced survivin-specific CTLs from Peripheral Blood Mononuclear Cells (PBMCs) efficiently recognized and lysed autologous mature DCs pulsed with the antigenic peptide or transfected with whole tumor RNA purified from a survivin-expressing cell line derived from primary autologous malignant CLL cells. In addition, survivin-specific CTLs were not able to lyse mature DCs or activate B and T cells 163. Specific T-cell reactivity against survivin-derived HLA-B35 restricted epitopes was also detected in the peripheral blood from patients with different malignancies, especially B-CLL, multiple myeloma and melanoma. Spontaneous T cell responses against survivin-derived peptides were found in 6 of 10 B-CLL patients 164. These findings raise the possibility that targeting survivin can be an innovative and efficient approach for designing potential protein- and peptide-based anticancer vaccines. Moreover, S12, a small molecule inhibitor of survivin, remarkably inhibited the growth of representative B lymphoma lines in vitro 165. Another small molecule inhibitor of survivin YM155 potentially suppressed proliferation and effectively induced apoptosis in the proliferative subset of CLL cells. Interestingly, YM155 treatment diminished anti-apoptotic proteins Mcl-1 and BCL-2 expression levels independently of the level of survivin expression which might pave the way for novel therapeutic applications of YM155 in treatment of CLL 166. A phase I/II clinical trial of terameprocol (NCT00664677), a novel survivin and cdc2/CDK1 Inhibitor, in patients with various advanced leukemias and hematological malignancies, including CLL showed that the drug was safe with a potential clinical activity 167.
FCRL; Fc receptor-like molecules: Fc Receptor-Like (FCRL) molecules, as novel members of the Immunoglobulin Superfamily (IgSF), are preferentially expressed by B-cells with potential activating and inhibitory roles 168. FCRL1-5 are dominantly expressed on developing B cells at different stages of development. But, FCRL3 and FCRL6 are especially expressed in different subsets of T and NK cells 168. Expression profiles of different FCRL family members have been investigated in autoimmune diseases, infectious diseases and B-cell malignancies 168,169. Interestingly, FCRL2 has shown 94% concordance with IgHV mutational status suggesting the importance of FCRL2 as a novel and potential prognostic biomarker in CLL. In addition, FCRL2 expression was also inversely associated with clinical progression in B-CLL 170. We showed elevated expression levels of CD305/LAIR-1 (leukocyte associated Ig like receptor 1) and FCRL2 in mutated compared to unmutated B-CLLs 171. High and exclusive expression levels of FCRL2 in CLL hold enormous potential to bring it as a novel diagnostic marker and therapeutic target in CLL patients.
Fibromodulin: Fibromodulin (FMOD) belongs to small leucine rich repeat (LRR) protein family, first identified as a 59 kDa collagen-binding protein with a broad range of tissue distribution 172. FMOD involved in regulation of collagen organization and assembly of matrix components by interaction with type I and type II collagen fibrils 173. Several independent GEF analyses have revealed aberrant expression of FMOD (287-fold) in leukemic B cells compared to normal B cells 144, 174, 175. Overexpression of FMOD in CLL B cells samples has also been confirmed at mRNA and protein levels 176,177. Analysis of FMOD revealed widespread cell surface expression of GPI-anchored FMOD in CLL patients compared to healthy individuals 178. Specific CTL responses have been identified against four different HLA-A2 binding FMOD derived peptides 176. A recent study demonstrated that T cells from CLL patients were able to precisely recognize FMOD overexpressing leukemic B cells and responded to naturally processed and presented HLA-A0201 binding peptides derived from FMOD. They also showed that FMOD specific CD8+ T cells derived from CLL patients could be expanded 176. Besides the possible role of FMOD in the pathophysiology of CLL, it could be a beneficial TAA candidate for immunotherapeutic intervention of CLL.
Bax and BCL-2: The ability of tumor cells to evade apoptosis is one of the hallmarks of human cancers. Evasion of apoptosis is often mediated by pro- and anti-apoptotic BCL-2 family proteins that are frequently highly expressed in cancers 179. Expression of Bax or Bak plays a critical role in suppressing cancer development and their reduced expression has been reported in several malignancies 180. Furthermore, proteasome-mediated degradation of Bax has been reported in advanced CLL that is associated with poor prognosis and chemoresistance of the disease 181. Generation of immunogenic peptides following proteasomal degradation of Bax might be related to immunologic consequences via presentation by MHC class I molecules. To address this hypothesis, Nunes et al showed that Bax peptide–specific T cells had been able to recognize and kill primary malignant cells from CLL patients 182. This finding suggests that generating Bax specific T cells for adoptive cell therapy protocols can be a promising therapeutic approach for treatment of CLL and other malignancies. It has been demonstrated that BCL-2 is an important protein in predicting survival in CLL. Increase in the BCL-2/BAX ratio in CD38 and CD49d positive patients was associated with resistance to apoptosis, and the clinical significance of this change was contributed to pathogenesis, chemorefractoriness and clinical outcome of CLL 183. Selective inhibition of BCL-2 by ABT-199 highlighted the potential role of BCL-2 family proteins in the context of targeted therapies 184. CD38 and CD49d overexpression are well known to be potential adverse prognostic markers in CLL 185-187. Administration of SPC2996, a novel BCL-2 mRNA antagonist, resulted in rapid leukemic cell clearance and immune activation in CLL patients 188. Phase I/II dose-escalating study of SPC2996 in 25 patients with relapsed CLL showed all six CLL patients in the treatment group with maximum drug concentration (4 mg/kg/dose) had a significant reduction in lymphocyte count with shrinkage of nodes at the higher tolerated doses 189. Currently, a large number of clinical trials assessing monotherapy and combination therapies, which are typically based on BCL-2 targeting, are underway. Venetoclax is first and only BCL-2 inhibitor approved for CLL as monotherapy or in combination with obinutuzumab or rituximab. A 4-year follow-up from a multicentre, open-label, randomised, phase 3 trial demonstrated that CLL patients treated with venetoclax–obinutuzumab or venetoclax–obinutuzumab– ibrutinib versus the chemoimmunotherapy, improved undetectable Measurable Residual Disease (MRD) rates and progression free survival 190.
CD229 (Ly9); a homophilic receptor: subset of the immunoglobulin superfamily 180. CD229 is expressed on thymocytes and mature T and B lymphocytes 191. Overexpression of CD229 has been clarified in all naïve CLL patients with stable expressions during the course of the disease 181. CD229-specific T cells presented a specific and strong killing activity against primary unmodified HLA-A0201+/ CD229+ B-CLL cells and T2 cells pulsed with synthetic peptides derived from CD229 protein in vitro 192. In addition, CD40L-stimulated B-CLL cells and native unmodified B-CLL cells as APCs were able to specifically expand antigen-specific autologous T cells from B-CLL patients 192. These results showed that CD229 is a naturally processed and presented antigen in B-CLL which can be considered a TAA in this disease. Thus, these findings provide strong evidence that CD229 can be an attractive target for the design and implementation of T-cell–based adoptive immunotherapeutic approaches for CD229-expressing malignancies including B-CLL.
FMNL1; a formin like protein 1: Formins are cytoskeleton-organizing proteins that play essential roles in cytokinesis and driving alterations in cell polarity, vesicular trafficking, signaling to the nucleus and embryonic development 193. Recently, a novel human gene, related to the formin family has been described. mRNA expression analysis demonstrated restricted expression of a formin like protein (FMNL1) in peripheral blood leukocytes, spleen, and thymus. However, at the protein level, low expression of FMNL1 was found to be restricted to lymph nodes and peripheral blood leukocytes. Interestingly, overexpression of FMNL1 was observed in Jurkat and Molt-4 cell lines and CLL tumor cells 194. This study also showed interaction between FMNL1 and Akt, suggesting a possible role for this protein in Akt signaling pathway 194. Another human leukocyte formin gene, termed KW-13, has been defined as a novel TAA overexpressed in CLL 195. Further research should clarify the exact role of formin protein family in CLL and also in immunotherapeutic based strategies.
RHAMM/CD168; the receptor for hyaluronan-mediated motility: Differential mRNA expression of TAAs in B-CLL patients has identified tumor-restricted antigen expression of RHAMM/CD168, fibromodulin, PRAME and MPP11 in CLL samples, but not in healthy donors. Higher expression levels of HSJ2 (100%), MAZ (93%) and OFAiLRP (100%) have also been detected in CLL patients. Analysis by conventional RT-PCR showed a more frequent expression of RHAMM/CD168 in advanced stages of CLL 196. RHAMM/CD168 expression was detected in both ZAP-70-positive and negative B-CLL patients. In addition, IFN-γ and granzyme-B secreting CD8+ T cells showed specific response against T2 cells pulsed with RHAMM/CD168 derived peptides 196. In another study, significantly increased specific CTLs against RHAMM/CD168 derived R3 peptide was detected after vaccination with DCs pulsed with CLL cell lysates 197. CD8+ T-cell responses against RHAMM/CD168 have also been described in AML patients 198. These results showed that targeting RHAMM/CD168 as a TAA may serve as an effective cell-based immunotherapeutic strategy for RHAMM/ CD168 overexpressing CLL patients.
hTERT; human telomerase reverse transcriptase: A functional catalytic protein subunit of a RNA-dependent DNA-polymerase, named human telomerase reverse transcriptase (hTERT), adds repeat sequences of DNA (TTAGGG) to the 3` end of chromosomes 199. Telomere length maintained by telomerase and ectopic telomerase expression leads to survival and unlimited proliferative capacity of malignant cells 200. High telomerase activity that is correlated with expression of hTERT has been demonstrated in more than 90% of all human tumors 200. High telomerase activity has also been reported in the majority of acute and chronic leukemias. Significantly higher levels of telomerase activity in various B-cell malignancies including CLL, Hairy Cell Leukemia (HCL) and Mantle Cell Lymphoma (MCL) have been detected in late stages than in early stages 201. In addition, the activity and therefore length of telomere might be associated with disease prognosis in B-CLL 201. hTERT overexpression was found in about 75% of CLL patients 202. DCs pulsed with a hTERT derived peptide (hTERT 611–626 peptide) were able to stimulate autologous T cells in hTERT expressing but not in hTERT-negative B-CLL patients and healthy control donors. Expanded autologous hTERT-specific cytotoxic T cells showed cytotoxic activities against hTERT overexpressing B-CLL cells in MHC class I-restricted manner 202. These findings suggest targeting hTERT as a suitable vaccine in B-CLL patients.
OFA-iLRP; oncofetal antigen immature laminin receptor protein: As a highly conserved protein, oncofetal antigen immature laminin receptor protein (OFA-iLRP) is preferentially expressed in fetal tissues and has been identified in many types of tumors 203, 204. Siegel et al demonstrated that OFA-iLRP could act as a potential target for T-cell-based immunotherapeutic approaches against hematologic malignancies such as lymphomas, AML and CLL 205. They primarily showed significant overexpression of OFA-iLRP in all hematologic tumor cell lines and all B-CLL and AML samples. OFA-iLRP was neither detectable in early stages of cancer nor in healthy donors. OFA-iLRP-specific CTLs were able to kill primary AML blasts, malignant B-CLL cells and OFA-iLRP-loaded T cells 205. Furthermore, it was also shown that mice treated with DCs transfected with OFA-iLRP-coding RNA, fully rejected the tumor and improved overall survival 205. Since OFA-iLRP mRNA was strongly expressed in PBMCs from healthy donors and B-CLL patients 196 as well as renal patients 206, it could not serve as a suitable target target for B-CLL immunotherapy.
SLLP1; sperm lysozyme-like protein 1: SLLP1 is a unique c-lysozyme-like protein predominantly expressed in the acrosome of human sperm 207. A study by Wang et al. showed that SLLP1 could serve as a novel cancer–testis antigen in some hematologic malignancies including AML, CLL, CML and multiple myeloma 208. In contrast to healthy donors, tumor cells of hematologic malignancies including AML, CML, CLL and multiple myeloma, showed aberrant expression of SLLP1. High titer IgG antibodies against SLLP1 were thus detected in the sera of malignant patients 208.
TCL1; T-cell leukemia/lymphoma 1: TCL1 onco-protein overexpression has been detected in many B-cell malignancies, including follicular lymphoma, CLL, MCL, diffuse large B-cell lymphoma, and splenic marginal zone B-cell lymphoma 209-212. TCL1-specific T cells were detectable in the peripheral blood and tumor-infiltrating lymphocytes of lymphoma patients. TCL1-specific T cells could also be expanded in an autologous setting and were able to recognize and lyse TCL165-79 peptide-pulsed T2 cells in MHC-I restricted manner 211. Therefore, TCL1 was demonstrated to be naturally processed and presented on the surface of primary human lymphoma cells to be recognized by CTLs and seems to be an ideal TAA that can act as a therapeutic target for development of new immunotherapeutic strategies against B-cell lymphomas especially for CLL patients 211.
Adipophilin: Adipophilin as a marker of lipid accumulation initially is involved in lipid storage and is considered to be expressed only in adipocytes 213, but it has been found to be expressed by a variety of cells including macrophages and tumor cells 214,215. Adipophilin derived peptides are capable of inducing effective adipophilin-specific CTLs in a wide variety of malignancies including renal cell carcinoma, breast cancer, melanoma, multiple myeloma, and primary autologous CLL cells or cells from plasma cell leukemia 216. Generated adipophilin-specific CTLs from PBMCs of CLL patients are able to recognize and lyse autologous RNA transfected DCs purified from adipophilin-positive tumor cell lines and autologous primary CLL cells, while nonmalignant B cells, T cells, monocytes, and DCs were unharmed 216. It may thus be suggested that adipophilin can act as an ideal candidate TAA candidate for development of CLL specific T-cell-based immunotherapy.
APRIL; a proliferation-inducing ligand and BAFF; B-cell activating factor: A proliferation-inducing ligand (APRIL) and B-cell Activating Factor (BAFF) belong to the Tumor Necrosis Factor (TNF) ligand family and are critical for the maturation, survival, and differentiation of normal and malignant B cells 217-219. BAFF and APRIL have been shown to play a crucial role in the pathogenesis and maintenance of B CLL tumor cells 220. Additionally, APRIL enhances tumor growth in human and murine tumor cell lines in vitro and in vivo 221. Interestingly, B CLL malignant cells express BAFF and APRIL and stimulation of these receptors promote CLL cell proliferation and survival in vitro 222,223. Additionally, BAFF and Aprill protected B-CLL cells against spontaneous and drug-induced apoptosis 220. Elevated BAFF levels are observed in patients with CLL, particularly those with unmutated IgHV and Higher BAFF expression is linked to unfavorable outcomes in these patients 224. Increased serum levels of BAFF (sBAFF) and APRIL (sAPRIL) are considered as potential predictive factors in B-CLL patients 225. Enhanced intracellular APRIL and BAFF levels within CLL cells correlate with elevated expression of unfavorable prognostic markers CD38 and ZAP70, and poorer clinical outcomes 224. BAFF, APRIL, and their receptors have therefore attracted great attention as potential targets for B-CLL therapy. BAFF-neutralizing antibody belimumab remarkably increased the sensitivity of the leukemic cells to all three small molecule inhibitors including B cell receptor inhibitors ibrutinib and idelalisib as well as the BCL-2 antagonist venetoclax 226. These findings show that BAFF neutralization using belimumab in combination with small molecule inhibitors can serve as a promising therapeutic strategy for patients with CLL. Belimumab is currently undergoing phase II clinical trials (NCT05069051) for the treatment of relapsed and/or refractory CLL 219. Patients who undergo treatment with CD19-targeting chimeric antigen receptor (CAR)-T cells for B-cell lymphoid leukemias and lymphomas experience relapsed and/or refractory (R/R) disease. CAR)-T cells targeting BAFF-R (BAFF-R CAR, also known as MC10029 CAR) showed significant in vitro and in vivo antigen-specific cytotoxicity against CLL cell lines and against CLL patients’ tumors, respectively 227.
Pim-1; provirus integration site for Moloney murine leukemia virus: Pim-1 is a highly conserved serine/threonine kinase that fine-tunes several cellular functions such as cell cycle, cell survival, drug resistance 228. Upregulation of Pin-1 expression has been reported in CLL compared with normal lymphocytes and PIM kinase inhibitors showed an effective therapeutic efficacy for CLL patients 229. HLA Ligandome Analysis showed the highly expression of SET nuclear proto-oncogene (SET), Pim-1 oncogene and Mucin 1 as new TAA in CLL cells 230. The SET oncoprotein is overexpressed in CLL cells, and SET levels are predictive of Overall Survival (OS) and the Time To Treatment (TTT) 231. Inhibition of SET protein leads to the enhance apoptosis, decrease Mcl-1 levels, and also is highly cytotoxic to malignant B cells in vitro and in vivo 231. These data show that targeting SET and Pim-1 could be a promising strategy to the treatment of CLL.
IGLV3-21; immunoglobulin lambda variable 3-21: The CLL B CLL subsets bearing IGLV3-21R110 BCR light chain represents an aggressive clinical course and serves as a poor prognostic factor in CLL patients 232. Surface expression IGLV3-21R110 neoantigen with oncogenic activity may be considered a potential target for CAR T cell therapy in CLL patients. New types of CAR-T cells with CAR construct of BCR light chain neoepitope composed of point mutation IGLV3-21R110 are able to selectively eradicate poor-risk subset of IGLV3-21R110 expressing cell lines and primary CLL cells 233.
Conclusion :
Tumor cells express a variety of poorly immunogenic antigens at different stages of cancer. However, identification of novel immunogenic CLL associated antigens that are generally expressed is essential to overcome the barriers of patient-specific idiotype vaccines. Furthermore, identification of human TAAs in cancer is developing as a critical part of clinical trials at this time (Table 2). mAbs exhibit an exciting addition to the growing list of agents that are used to treat CLL. Molecules that are tumor-specific or overexpressed in cancers may play fundamental roles to contribute to tumor cell development, cellular transformation, and migration. Today, new promising molecules for CLL are being tested in clinical trials. Targeting of such molecules can promote the anti-tumor effect and therefore might be valuable approaches for cancer therapy. Application of specific mAbs to target certain antigens have shown great potentials as valuable approaches in preclinical and clinical investigations and will thereby continue to revolutionize the treatment of CLL as we know it today. Malignant CLL cells exhibit features of activated and antigen-experienced B cells. Constitutive and stable expression of specific targets on malignant B cells and natural processing and presentation of these molecules on the surface of lymphoma cells for recognition by cytotoxic T cells as TAA in primary B-CLL, enabling the expansion of autologous tumor-specific T cells, can serve as novel targets for development of immunotherapeutic strategies against common B-cell malignancies. Recently there has been significant interest in multiple TAA as therapeutic targets in CLL patients as evidenced by several studies that have demonstrated the existence of autologous T cells against TAAs which functionally respond with IFN-γ secretion after recognition of these antigens. These findings are promising in the identification of specific target-derived peptides as TAAs in B-CLL that open numerous pathways to consider these molecules for therapeutic interventions in this disease. However, preclinical studies are essential to achieve the optimal vaccine formulation and identify the safety and efficacy of TAA-derived vaccines before evaluation in clinical trials.
Conflict of Interest :
The authors have no financial conflicts of interest.
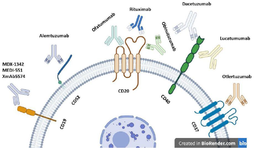
Figure 1. Tumor associate antigens targeted for monoclonal antibody-based immunotherapy of chronic lymphocytic leukemia.
|
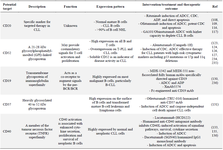
Table 1. Approved monoclonal antibodies targeting tumor antigens in CLL patients
ADCC: Cytotoxic effect mainly by antibody dependent cellular cytotoxicity, CDC: Complement Dependent Cytotoxicity, ADP: Antibody Dependent Phagocytosis, CLL: Chronic Lymphocytic Leukemia, T-PLL: T Cell Prolymphocytic Leukemia.
|
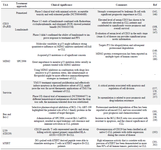
Table 2. Tumor antigens with potential treatment utility in CLL patients
TAA: Tumor associated antigen, PFS: Progression-Free Survival, MDM2: Mouse Double Minute 2 homolog, hTERT: human Telomerase Reverse Transcriptase, PFS: Progression-free survival, CLL: Chronic Lymphocytic Leukemia.
|
|