Lippia multiflora Leaves Extracts Enhance Cefotaxime Bactericidal Effects and Quench the Biofilm Formation in Methicillin-Resistant Staphylococcus aureus ATCC 43300
-
 Rouamba , Ablassé
Laboratory of Applied Biochemistry and Chemistry, UFR SVT, Université Joseph Ki-Zerbo, 03 BP 7021, Ouagadougou 03, Burkina Faso, Tel: +226 70797736; E-mail: rouambaablasse@gmail.com
Rouamba , Ablassé
Laboratory of Applied Biochemistry and Chemistry, UFR SVT, Université Joseph Ki-Zerbo, 03 BP 7021, Ouagadougou 03, Burkina Faso, Tel: +226 70797736; E-mail: rouambaablasse@gmail.com
-
Ecole Normale Supérieure, 01 BP 1757, Ouagadougou 01, Burkina Faso
-
Badini, Djaouratou
-
Laboratory of Applied Biochemistry and Chemistry, Department of Biochemistry-Microbiology, UFR SVT, Université Joseph Ki-Zerbo, 03 BP 7021, Ouagadougou 03, Burkina Faso
-
Compaoré, Eli
-
Laboratory of Applied Biochemistry and Chemistry, Department of Biochemistry-Microbiology, UFR SVT, Université Joseph Ki-Zerbo, 03 BP 7021, Ouagadougou 03, Burkina Faso
-
Ouédraogo, Vincent
-
Laboratory of Applied Biochemistry and Chemistry, Department of Biochemistry-Microbiology, UFR SVT, Université Joseph Ki-Zerbo, 03 BP 7021, Ouagadougou 03, Burkina Faso
-
Kiendrebeogo, Martin
-
Laboratory of Applied Biochemistry and Chemistry, Department of Biochemistry-Microbiology, UFR SVT, Université Joseph Ki-Zerbo, 03 BP 7021, Ouagadougou 03, Burkina Faso
Abstract: Background: The emergence of the multidrug-resistant bacteria strain has become a global world crisis. This study was designed to evaluate the antibiofilm and synergistic effects of Lippia multiflora (L. multiflora) leaf extracts on the activity of cefotaxime against the methicillin-resistant Staphylococcus aureus (S. aureus).
Methods: The synergistic effect of methanol and dichloromethane extracts on the bactericidal activity of cefotaxime was determined by using the antibiotic susceptibility test on agar medium. The antibiofilm activity of the extracts was measured by using the crystal violet method. The antioxidant potential of the extracts was assessed by using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and Ferric Reduction Activity Potential (FRAP) methods. The main secondary metabolites groups were analyzed by using different standard analytical tests. The total phenolics and total flavonoids were quantified spectrophotometrically.
Results: The methanol extract (final concentration of 100 µg/ml) inhibited the formation of bacterial biofilm more than salicylic acid (p<0.05). All extracts combined with cefotaxime (20 µg and 200 µg) showed good synergistic bactericidal effect on S. aureus with inhibitory diameters of up to 40 mm. The methanol extract showed higher total phenolics (462.20±10.90 mg EAG/g) and total flavonoids (26.20±0.20 mg EQ/g) contents than the dichloromethane extract (96.70±1.70 mg EAG/g and 8.00±1.20 mg EQ/g). Moreover, the methanol extract showed a higher FRAP reducing power (353.6± 4.17 mmol EQ/g) than the dichloromethane extract (385.3±7.01 mmol EQ/g). Qualitative phytochemical analysis showed the presence of tannins, flavonoids, terpenes and sterols in both extracts.
Conclusion: These data showed that L. multiflora leaves contain effective antibacterial phytomolecules for combating bacterial resistance.
Introduction :
The bacterial resistance to antibiotics has become a major global concern 1. Nowadays we are witnessing the emergence of numerous bacterial strains that are less sensitive or insensitive to the conventional antibiotherapy 2. Staphylococcus aureus (S. aureus) is a Gram-positive bacterium, an opportunistic pathogen of humans and many animal species that colonizes the skin and mucous membranes (nasal passages, perineum, gastrointestinal tract and pharynx) 3. In humans, it is responsible for numerous infections ranging from benign skin infections to deep, life-threatening infections, such as septicemia, endocarditis or even necrotizing pneumonia 4. S. aureus represents a public health challenge due to its great diversity of infections, its severity, its frequency and also due to the emergence of strains that are developing multi-resistance to antibiotics 5.
For benefit from a broad spectrum of infections, S. aureus is capable to produce a large number of virulence factors including exotoxins (hemolysins, leukocidins, enterotoxins, exfoliatins), enzymes (catalase, coagulase, deoxyribonucleases, phosphatases, hyaluronidases, fibrinolysins, lipases and proteolysins) and numerous adhesins (fibronectin binding proteins A and B, collagen binding proteins) specifically regulated by various molecules such as transcription factors, two-component systems and regulatory RNAs 6,7. Other studies have shown that S. aureus was capable of producing persistence factors such as biofilm formation, thus escaping the host cell's immune response and the bactericidal action of antibiotics 8. The bacteria surround themselves with a polysaccharide membrane poly-N-acety-glucosamine which is impermeable to antibiotics and form a coordination complex for the production of multiple virulence and adaptation factors 9. Biofilm biosynthesis is defined by 3 key stages such as a primary attachment, a maturation and a detachment to form new infectious foci. A large number of factors expressed by S. aureus are essential at each stage to allow the development of this complex structure 10. The resistance of S. aureus to antibiotics of the β-lactam family such as cefotaxime, methicillin, penicillin is mainly due to the enzymatic inactivation of these antibiotics by β-lactamases produced by the bacterium and the formation of biofilm which limits this antibiotics penetration 11. In front of the emergence of these bacterial strains that have developed a multi-resistance to the conventional antibiotic therapy, it therefore becomes necessary to find new more effective molecules which could combat this bacterial resistance alone or in synergy with antibiotics.
Lippia multiflora (L. multiflora) is an aromatic plant of the Verbenaceae family (Figure 1) 12. It is native to Africa, where it is widely distributed, particularly in Sub-Saharan and tropical Africa. In West Africa, the infusion of the leaves is used in the folklore medicine to treat fevers, gastrointestinal disorders, enteritis, coughs, colds, jaundice, stomach aches, lung infections and oral candidiasis 13. The present study aims to evaluate the antibiofilm potential of dichloromethane and methanol extracts of L. multiflora leaves as well as their synergistic effects on the bactericidal activity of cefotaxime in S. aureus ATCC 43300.
Materials and Methods :
Chemicals: All reagents were analytical grade. Ascorbic acid, gallic acid, quercetin, sodium carbonate, folin-ciocalteu reagent, cristal violet, aluminium chloride, Dimethyle Sulfoxide (DMSO), Diphenylpicryl-hydrazine (DPPH), acetic acid, Luria Bertani (LB) and agar were purchased by Sigma Aldrich, Germany. Methanol and dichloromethane were purchased by ProLabo, France.
Plant collection: The leaves of L. multiflora were freshly collected in September 22, 2022 at Loumbila, a locality situated at the northeast of Ouagadougou (12°31’5.39’’N;-1°22’8.39’’W). The identification of the plant was carried out by Professor Amadé OUEDRAOGO, a full Professor in Biology and Plant Ecology at the UFR/SVT, University Joseph KI-ZERBO. An herbarium was created using a leafy stem of the plant and registered at the UFR/SVT herbarium under the identification code CI-922.
Extraction: The leaves of L. multiflora were dried in the laboratory at room temperature (37°C) and powdered. 50 g of the powder was macerated successively in 500 ml of dichloromethane and in 500 ml of methanol (37°C, 24 hr) under magnetic stirring. The solutions obtained were filtered with the Whatman filter paper (pore 0.2 µm) and the filtrates were concentrated in a rotary vapor (Büchi, Germany) and carried out to dryness allowing to obtain the dichloromethane extract (E_DCM) and the methanolic extract (E_MeOH). These extracts were stored at 4°C for phytochemical and biochemical investigations.
Phytochemical investigations: The presence of the main families of secondary metabolites in the extracts was analyzed by using standard analytical phytochemical methods 14.
The total phenolic was quantified spectrophotometrically at 760 nm by using the Folin-Ciocalteu colorimetric assay 15. Gallic acid was used to generate the standard curve (y=39.543x+0.039; R²=0.9998; p< 0.001). The total flavonoid content of extracts was measured spectrophotometrically at 415 nm by using the aluminim chloride as described previously 16. Quercetin was used to generate the standard curve (y=0.0309x+0.0186; R²=0.9999; p<0.001) as described previously.
Antioxidant activity: The antioxidant properties of the extracts were evaluated by measuring the ability of the extracts to trap the free radical DPPH or reduce the ferric ion Fe3+ according to the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and Ferric Reduction Activity Potential (FRAP) methods 17. For the DPPH assay, 200 µl of the DPPH solution were incubated with 100 µl of extract for 15 min and the optical density of the mixture was measured at 517 nm. Regarding to the FRAP assay, extract was incubated with K3Fe(CN)6 for 20 min at 50°C. The chloride acetic acid and the iron chloride were added. The optical density was measured at 700 nm. Ascorbic acid was used to generate the standard curve (y=0.058x+0.1297; R²=0.9946; p<0.001).
Antibiofilm activity: Determination of inhibitory and bactericidal minimum concentrations: in order to use non-bacteriostatic and non-bactericidal concentrations of the extracts to measure the antibiofilm capacity of the extract, the Minimum Inhibitory Concentration (MIC) and the Minimum Bactericidal Concentrations (MBC) of the extracts were determined using the antibiotic susceptibility test on the LB broth and the LB-agar respectively 18.
Antibiofilm assay: the antibiofilm activity of the extracts was measured by the crystal violet method by using a non-bactericidal and non-bacteriostatic concentration of the extracts as described previously 19. Briefly, 100 µg/ml of each extract were incubated for 24 hr at 37°C in the presence of the S. aureus inoculum. The planktonic bacteria were removed with the supernatant and the developed biofilms were strained with the crystal violet for 30 min. The excess of the crystal violet was removed and a solution of acetic acid was added to dissolve the crystal violet fixed by the biofilm. The optical densities that are proportional to the amount of the biofilm were measured at 590 nm. The results were expressed as inhibition percentages of biofilm formation compared to a vehicle (DMSO 1%) according to the equation 1. Salicylic acid was used as a reference compound.
Equation 1: AA= A1-A0A1
AA: Antibiofilm activity (%)
A1: Absorbance of DMSO 1% at 590 nm
A0: Absorbance of extract or salicylic acid at 590 nm
Synergistic bactericidal effect of extracts and cefotaxime: To measure the synergistic effect of the extracts on the bactericidal activity of cefotaxime on S. aureus, the inhibition diameters of the bacterial growth on agar medium were measured as described previously 20. Discs of 6.5 mm diameter were impregnated with cefotaxime alone (20 and 200 µg), each extract alone (20 and 200 µg) or the extract-cefotaxime combination (1:1 w/w) and placed on an agar medium previously spread with the bacterial inoculum. After 24 hr of incubation at 37°C, the inhibition zones of the bacterial growth were photographed and their diameters were measured.
Statistical analysis: The results were expressed as mean values of several independent experiments (n=6) ± standard deviation. One Way ANOVA analysis of variances followed by the Newman Keuls post-test were used to verify the degree of significance between the different treatments. A statistical difference was considered at p<0.05.
Results :
The antibiotic susceptibility test on the LB broth medium allowed to record a MIC that was 12.5 mg/ml and greater than 50 mg/ml for the methanol extract and the dichloromethane extract respectively (Table 1). The methanolic extract was therefore more bacteriostatic than the dichloromethane extract. Likewise, the methanol extract (MBC=25 mg/ml) was more bactericidal than the dichloromethane extract (MBC >50 mg/ml) (p<0.05).
In its pathogenicity, S. aureus generates oxidative stress. The antioxidant properties of the extracts were evaluated and the results were recorded in the table 1. The methanol extract showed the highest FRAP reducing activity (p<0.05) while the methanol and the dichloromethane extracts showed a similar anti-DPPH activity (p>0.05).
To assess the antibiofilm effect of the extracts, a non-bacteriostatic and non-bactericidal concentration of extracts (100 µg/ml) lower than the MIC was used and the results are shown in figure 2. The methanol extract showed the good antibiofilm activity than the dichloromethane extract and the salicylic acid (p<0.001) with an inhibition percentage more than 50%. These results suggested that the antibiofilm compounds contained in the L. multiflora leaves are mainly polar, extractable by the methanol.
To observe the synergistic effect of the extracts on the bactericidal activity of cefotaxime on S. aureus, the bacterial inoculum was incubated in the presence of the extract alone, the antibiotic alone or the extract combined with the antibiotic and the inhibition zones of the bacterial growth were photographed (Figure 3). The analysis of the different inhibition zones showed that when the bacteria is incubated with the antibiotic alone or with the extract alone, the inhibition zone of the bacterial growth was restricted. However, when the bacteria were incubated both in the presence of the extract and the antibiotic, the inhibition zones of the bacterial growth were much larger, suggesting a synergistic effect of the extract on the bactericidal activity of the antibiotic.
The diameters of the inhibition zones of the bacterial growth in the presence of cefotaxime alone, the extract alone or the antibiotic-extract combination are shown in the figure 4. The data showed that the antibiotic alone or the extract alone weakly inhibited bacterial growth with inhibition diameters less than 20 mm (20 µg as final concentration) and inhibition diameters less than 30 mm (200 µg as final concentration). Nevertheless, when the bacteria were incubated in the presence of both the extract and the antibiotic, the inhibition diameters increased significantly compared to the antibiotic treatment alone or the extract treatment alone (p<0.001). These data showed that the extracts exerted a synergistic effect on the bactericidal activity of cefotaxime on the S. aureus growth. The methanolic extract presented a higher synergistic bactericidal effect (inhibition diameter up to 40 mm) than the dichloromethane extract (p<0.05). The synergistic effect of the extracts on the bactericidal activity of cefotaxime was not concentration dependent in the concentration range used in this study.
The phytochemical analysis of the extracts was carried out qualitatively and quantitatively and the results were presented in the table 2. The qualitative phytochemical analysis showed the presence of tannins, sterol and terpenes in all the extracts. However, the methanolic extract contained quinones unlike the dichloromethane extract. The presence of alkaloids was not detected in both extracts. The quantitative phytochemical screening showed that the methanol extract had higher total phenolic and total flavonoid contents than the dichloromethane extract (p<0.05).
Discussion :
Bacteria under biofilm are 1000-fold more virulent than their planktonic form. The virulence of S. aureus is due to its ability to adhere to a surface and form a biofilm 21. The methanol extract of L. multiflora leaves showed in this study a greater antibiofilm potential than salicylic acid. The antibiofilm effect of this extract would be due to its inhibitory effect on the secretion of adhesins by the bacteria, necessary for the primary attachment and maturation of the biofilm. The extract could also inhibit the synthesis of the polysaccharide matrix of the biofilm or cause its disruption. The regulatory system (quorum sensing) of the different stages of the biofilm formation could also be targeted by the bioactive secondary metabolites present in the extract. The methanol extract showed high contents of total flavonoids and total phenolic compounds, which could be responsible for the antibiofilm effect of the extract. Indeed, flavonoids such as myricetin, hesperetin and phloretin inhibited S. aureus biofilm formation by repressing the expression of the efflux protein genes 22. The Biofilm Associated Protein (BAP), that mediated the S aureus biofilm matrix synthesis is quenched by flavonoids compounds such as quercetin, myricetin and scutellarein 23. Others studies showed that phenol acids compounds can cause S aureus membrane wall damage and the inhibition of Na+/K+-ATP-ase activity leading to biofilm matrix disruption 24. Moreover, the analytical phytochemical investigations of the methanol extract showed the presence of tannins, quinones and terpenes and their antibiofilm properties have been demonstrated in previous studies. Indeed, tannic acid inhibited S. aureus biofilm formation through the inactivation of the enzyme transglycosylase IsaA 25. Quinones inhibited the efflux pump system in methicillin-resistant S. aureus strains and quenched the quinone oxidoreductase WrbA that potentially modulate the biofilm formation and maturation 26. The combinaison of terpenes [(-)-Trans-Caryophyllene and Linalool] inhibited the biofilm formation in S. aureus by targeting the initial adhesion of the biofilm 27. S. aureus in its pathogenicity induces the release of cytokines and interleukins leading to an inflammation, an oxidative stress and the increasing in immune cells apoptosis 28. The extracts exhibited in this study an antioxidant potential through the inhibition of the DPPH radical and the reduction of Fe3+. These antioxidant properties of the extracts could be beneficial in patients under S. aureus infections.
The bactericidal effects of antibiotics are due to several mechanisms such as an inhibition of the cells wall synthesis, a destruction of the cytoplasmic membrane, an inhibition of the proteins or nucleic acids synthesis 29. β-lactam antibiotics such as cefotaxime are inhibitors of bacterial wall synthesis 30. These antibiotics are deactivated enzymatically by β-lactamases produced by S. aureus which has become insensitive or less sensitive to many β-lactam antibiotics 31. In this study the extracts presented an interesting synergistic effect on the bactericidal activity of cefotaxime against the growth of S. aureus. The extracts could induce the alteration in the metabolic activity of the bacteria, thus modifying their sensitivity to cefotaxime. The extracts, by inhibiting the expression of genes coding for the β-lactamases synthesis or by directly inhibiting the enzymatic activity of β-lactamases, could restore the bactericidal activity of cefotaxime against S. aureus. Indeed, flavonoids such as epicatechin and naringin are uncompetitive inhibitors while catechin, morin and rutin are competitive inhibitors of β-lactamases 32.
Conclusion :
The methanol extract showed the best antibiofilm activity and the best synergistic effect on the bactericidal activity of the cefotaxime on S. aureus. The antibacterial potential of the methanol extract would be assigned to the secondary metabolites present in the extract. By inhibiting biofilm formation and restoring cefotaxime activity, the methanol extract of L. multiflora leaves would promote new strategies to combat effectively the bacterial resistance.
Ethical approval :
Not applicable.
Acknowledgement :
All authors would like to thank the "Fond National de la Recherche et de l’Innovation pour le Développement (FONRID)" for funding this research and the laboratory of bacteriology of the "Centre muraz de Bobo Dioulasso" for providing the S. aureus ATCC 43300 strain.
Funding: This present work was funded by the "Fond National de la Recherche et de l’Innovation pour le Développement (FONRID)" under the reference FONRID//AAP3/Malainfect/NCP/PC/2021.
Conflict of Interest :
All authors declared that there is no conflict of interest.

Figure 1. Lippia multiflora MOLDENKE.
|
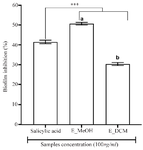
Figure 2. Antibiofilm activity of plant extracts.
*** p<0.001 versus salicylic acid (ANOVA followed by Newman Keuls post-test); histograms with different superscript letter (a, b) were statically different at p<0.05 (ANOVA followed by Newman Keuls post-test). E_MeOH: Methanol extract; E_DCM: Dichloromethane extract.
|
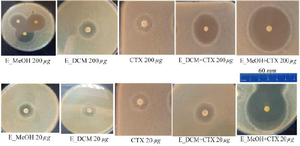
Figure 3. Inhibition zones of the different treatment.
CTX: Cefotaxime; E_MeOH: Methanol extract; E_DCM: Dichloromethane extract.
|
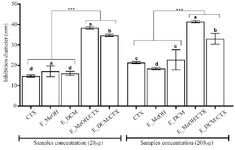
Figure 4. Inhibition diameters of the different treatment.
*** p<0.001 versus cefotaxime or extracts alone (ANOVA followed by Newman Keuls post-test); histograms with different superscript letter (a, b, c, d) were statically different at p<0.05 (ANOVA followed by Newman Keuls post-test).
CTX: Cefotaxime; E_MeOH: Methanol Extract; E_DCM: Dichloromethane Extract.
|
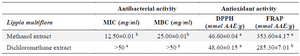
Table 1. Antibacterial and antioxidant activities
Values with different superscript letter (a, b) differ statically at p<0.05 for each parameter considered (ANOVA followed by Newman Keuls post-test).
MIC: Minimal Inhibitory Concentration; MBC: Minimal Bactericidal Concentration; AAE: Ascorbic Acid Equivalent.
|

Table 2. Phytochemical screening of extracts
Values with different superscript letter (a, b) differ statically at p<0.05 for each phytochemical quantification (ANOVA followed by Newman Keuls post-test).
TFC: Total Flavonoids Content, TPC: Total Phenolics Content; QE: Quercetin Equivalent; GAE: Gallic Acid Equivalent; (+): presence; (-): absence
|
|