Exploring the Molecular Underpinnings of Skin Regeneration and Wound Healing: The Role of Renin Angiotensin
-
Qoreishi , Seyedeh Hoda
-
Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
-
USERN Office, Mazandaran University of Medical Sciences, Sari, Iran
-
 Khazeei Tabari , Mohammad Amin
Mazandaran University of Medical Sciences, Sari, Iran, Tel: +98 9118228029; Fax: +98 11 33352725; E-mail: aminkhazeeitabari@gmail.com
Khazeei Tabari , Mohammad Amin
Mazandaran University of Medical Sciences, Sari, Iran, Tel: +98 9118228029; Fax: +98 11 33352725; E-mail: aminkhazeeitabari@gmail.com
-
USERN Office, Mazandaran University of Medical Sciences, Sari, Iran
-
Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran
-
Găman , Mihnea-Alexandru
-
Faculty of Medicine, “Carol Davila” University of Medicine and Pharmacy, 050474, Bucharest, Romania
-
Department of Hematology, Center of Hematology and Bone Marrow Transplantation, Fundeni Clinical Institute, 022328, Bucharest, Romania
-
Kazeminejad , Armaghan
-
Department of Dermatology, Antimicrobial Resistance Research Center, Communicable Diseases institute, Mazandaran University of Medical Sciences, Sari, Iran
Abstract: The aim of this study is to review the role of renin-angiotensin in skin regeneration and wound healing with a focus on molecular mechanisms. Angiotensin receptor type 1 (AT1R) are abundant in the wounded area, and thus, lead to the activation of ERK, STAT1, and STAT3 which can lead to epidermal self-renewal. The expression of Renin Angiotensin System (RAS) components was significantly lower in wounds caused by burning, rather than intact skin, noting that RAS is involved in the re-epithelialization of skin. ERK, STAT and STAT3 are the targets of Ang II, indicating that RAS active components are involved in fibroblast, stem cells and keratinocyte migration. The effect of inhibiting the RAS on wound healing is context-dependent. On one hand, it is suggested that inhibiting RAS during this phase may slow down wound healing speed. On the other hand, studies have shown that RAS inhibition in this phase can lead to α-SMA activation, ultimately accelerating the wound healing process. Most of the investigations indicate that the inhibition of RAS with Angiotensin Receptor Blockers (ARBs) and Angiotensin Converting Enzyme (ACE) plays a significant role in tissue remodeling in the last phase of wound healing. It has been shown that the inhibition of RAS can inhibit scar formation and fibrosis through the downregulation of inflammatory and fibrogenic agents, such as TGF-β, SMAD2/3, and TAK1, PDGF-BB, and HSP47. To sum up, that local administration of RAS regulators might lead to less scar formation and inflammation in the last phase of wound closure.
Introduction :
Restoring organ function following injury requires tissue regeneration 1. Wound healing is the most relevant mechanism of tissue regeneration in the human body 2. It takes blood, parenchymal cells, soluble mediators, and significant extracellular matrix remodeling for a wound to heal 3. An initial phase of wound healing contains a hematological response that involves hemostasis and an inflammatory response protecting the organism against pathogen invasion 4. A second phase contributes to tissue regeneration, and the third phase consists of a tissue remodeling process 3,5. In the final stage of wound healing, cell reduction and significant extracellular matrix regeneration support the differentiation of the keratinocyte layer and the turnover of dermal granulation tissue, thus regaining skin integrity and physiological function 6,7.
The Renin Angiotensin System (RAS) is a complex regulatory mechanism in the human body that controls blood pressure and fluid balance. It involves the sequential conversion of renin, released by the kidneys, to Angiotensin I (Ang I), which is then transformed into the biologically active Angiotensin II (Ang-II) by Angiotensin-Converting Enzyme (ACE) 8. Ang-II acts as a potent vasoconstrictor, leading to increased blood pressure and regulating fluid and electrolyte balance, making the RAS a critical component of cardiovascular homeostasis and a target for medications used to manage blood pressure 9. Ang-II is a peptide composed of eight amino acids derived from angiotensinogen by renin and ACE. Ang-II exerts opposite effects via two distinct G protein-coupled receptors, the AT1R& AT2R 10. Ang-II, the primary effector hormone of the RAS, is synthesized from its source, angiotensinogen, by the enzymes renin and ACE, with Ang-I as an intermediate, inactive result. Ang-II, an octapeptide generated mainly through proteolytic cleavage of its precursor Ang-I by ACE, has several physiological effects 11,12, including hormone secretion, tissue growth, and neuronal activity. Ang-II has been identified as a modulator of fibrosis in various tissues, and it has been demonstrated that Ang-II is a potent agent for accelerating wound regeneration 13,14. Several research projects have focused on localized RAS in skin over the two passed decades. According to available evidence, components of RAS significantly influence wound healing, tissue regeneration pathophysiology and scar formation 15.
The cardiovascular and renal effects of the RAS primary effector hormone, Ang-II, have been the focus of most documented research on RAS. Ang-II regulates systemic blood pressure specifically through the two receptor subtypes angiotensin receptor type 1 and 2 (AT1R and AT2R) 16. Ang-II blockers are utilized in clinical practice as anti-hypertensive agents. According to clinical data, subjects who are prescribed AT1R inhibitors have a lower risk of developing cardiovascular and renal diseases 17.
Ang-II governs cell migration and proliferation, as well as collagen metabolism, in addition to its role in blood pressure management, all of which are necessary steps in wound healing 18. RAS components are expressed in the skin, and are involved in various processes such as skin development, homeostasis, and wound healing. Studies have shown that various components of the RAS, including ACE, Ang-II and its receptor (AT1R), are expressed in the epidermis, dermis and hair follicles 19,20. Studies investigating the role of RAS expression in skin tissue suggest that the skin can be affected by RAS, although the relevance of this influence to skin health may not be clearly established or may not directly align with RAS activity in other organs 21. Despite this obstacle, since the initial discovery of RAS components expression in rat and human skin, the number of related publications has constantly risen. These studies have provided substantial findings 22. The research has shown that RAS plays a role in various aspects of skin physiology and pathology, including stem cell proliferation and differentiation, inflammation, scar and fibrosis formation, vascular motility, and skin cancer development 15. In the initial phase of wound healing, increased vascular permeability attracts leukocytes for inflammation, while epithelial stem cells and dermal fibroblasts are mobilized. ACE facilitates stem cell migration by converting Ang-I to Ang-II, laying the groundwork for regeneration 3. In the regeneration phase, Ang-II binds to AT2R, triggering the ERK/STAT1-3 pathway, boosting fibroblast proliferation and granulation tissue formation. The RAS also augments scar-forming molecules, promoting fibroblast proliferation and collagen accumulation in the healing process 23,24 (Figure 1).
In this manuscript, we reviewed the importance of RAS in skin physiology, regeneration of epidermal and stem cells, wound healing, and scar formation. Moreover, the role of Angiotensin Receptor Blockers (ARBs) is discussed in this review.
What is the role of RAS in skin physiology?: RAS consists of various components, with Ang-II being a central player. Ang-II is generated through a series of enzymatic reactions and acts as a potent vasoconstrictor, regulating blood pressure and fluid balance. In contrast, non-Ang-II components encompass other elements within the RAS, such as renin, angiotensinogen, Ang I, angiotensin receptors like AT1R and AT2R, and various peptides and enzymes. These components collectively contribute to the complex regulation of blood pressure, electrolyte balance, and vascular homeostasis, extending their influence beyond the actions of Ang-II and playing essential roles in maintaining physiological equilibrium and responding to changes in the body's hemodynamic conditions 25. Both non-Ang-II and Ang-II components of the RAS are closely related to G Protein-Coupled Receptors (GPCRs). Ang-II interacts with angiotensin receptors, particularly AT1R and AT2R, both of which are GPCRs. When Ang-II binds to these receptors, it initiates intracellular signaling through G proteins, leading to various physiological responses, including vasoconstriction and aldosterone release. Non-Ang- II components of the RAS, such as renin, also interact with GPCRs to trigger signaling cascades that ultimately impact blood pressure regulation, electrolyte balance, and vascular homeostasis. GPCR-mediated signaling is a fundamental mechanism in the complex regulation of the RAS and its effects on various physiological processes 26.
Although a full RAS in the skin was identified in rats (1994) and in humans (2004); the physiological function of RAS in the skin remains poorly known. AT1R and AT2R are the primary Ang-II receptors. The AT2R mediates vasodilation and natriuresis, opposing the AT1R’s cardiovascular activities, including vasoconstriction and sodium/water retention. Remarkably, RAS hormones and receptors affect inflammation, fibrosis, proliferation, differentiation, immunological response, and cardiovascular function 27.
Overstimulated AT1R causes inflammation and fibrosis. AT2R-mediated actions are primarily anti-inflammatory and anti-fibrotic, supporting the idea that AT2R is potential tissue protection agent 28-30. Surprisingly, all physiologically active, "non- Ang-II" angiotensin fragments acting on their receptors, such as the AT2R, mediate a series of protective activities, making this RAS axis the protective arm. Several researchers have also noted that rodents and human skin express all RAS components 18,31,32.
RAS constitutes are found in the dermal, epidermal, subcutaneous fat, micro vessels, sweat glands, sebaceous, and hair follicles 32-34. Antibodies used for the identification of angiotensin receptors impose limitations on various research endeavors. Specifically, the specificity of anti-AT1R and anti-AT2R antibodies, akin to many GPCRs antibodies, has raised concerns regarding their selectivity for their intended targets 35,36. Notably, some studies have shown that keratinocytes synthesize Ang-II (and probably other angiotensin) independently from the delivery of RAS components in the skin by the circulation, as shown in the first investigations establishing the presence of a local RAS in the skin 16,32. The regulatory mechanisms that govern this locally-produced Ang-II are unknown. However, skin-derived Angiotensin II contributes to the RAS's stabilizing impact on the circulation in cases of an abrupt decrease in blood pressure or acute, substantial volume loss or whether the cutaneous RAS is activated independently from the systemic RAS.
Jiang et al recently conducted research on the importance of RAS in skin physiology and found that Ang-II stimulates the growth of keratinocytes from mesenchymal stem cells derived from the bone marrow (BMdSC) in normal conditions. According to the findings of Jiang et al, Ang-II's effect on the differentiation of BMdSC into keratinocytes is mediated through Janus kinase 2/3, p38 MAPK, and c-Jun N-terminal kinase (JNK). This conclusion was reached as the differentiation was blocked when JNK and MAPK inhibitors like SB203580 and SP600125 were used. The authors claim that this effect is based on the presence of AT1R, though they did not provide any supporting evidence in their publication 37.
A further physiological function of the dermal RAS might be connected to the discovery that the skin is a crucial environment for sodium storage, which influences blood pressure control 38. A function for the RAS in altering cutaneous sodium storage is probable but has not been experimentally shown. Nonetheless, animal studies support the fact that skin affects physiological blood pressure control. These mechanisms, include the production of vasodilatory nitric oxide by keratinocytes, which is under the control of UV-light 39, a balance between HIF-1α, HIF-2α 40, and the local RAS. The relationship between the RAS and HIF is complex and multifaceted, involving both direct and indirect interactions. Both RAS and HIF play critical roles in regulating various physiological processes, and their interplay has been the subject of research in different contexts. Directly, Ang-II, a component of RAS, can influence the stability and activity of HIF-1, a transcription factor involved in cellular responses to hypoxia. Indirectly, RAS affects tissue oxygenation through its influence on blood pressure and vascular tone, impacting the need for HIF-mediated responses in hypoxic conditions. Both pathways are involved in tissue repair, inflammation, and various disease states, and their interactions hold therapeutic implications, particularly in conditions where both systems are dysregulated 41-43.
Regenerative role of renin angiotensin in epidermal cells: Epidermal cell regeneration, a fundamental biological process, encompasses the continuous renewal of the epidermis. This intricate mechanism relies on the division of basal cells, residing in the basal layer of the epidermis, which serve as stem cells responsible for generating new keratinocytes. Ultimately, these keratinocytes undergo a process known as cornification, leading to cell death, and their remnants are subsequently shed from the skin's surface during desquamation. This perpetual process ensures that the skin remains an effective protective barrier, while also playing a pivotal role in wound healing by rapidly regenerating skin in response to injuries 44. While much of this regenerative process is well-documented, it is essential to delve deeper into the intricate molecular mechanisms that govern epidermal cell renewal. Of particular interest is the RAS, which has long been known for its role in regulating blood pressure, but also exhibits a profound impact on tissue remodeling and cell proliferation 45. The critical role of RAS constitutes, such as ACE and ang-II receptors has long been known 32.
ACE is a highly important modulator of skin regeneration and cellular survival 46. ACE expression in Epidermal Stem Cells (ESCs) is associated with the expression of ESCs biomarkers, including beta integrins 47. ACE activity inhibition can postpone skin regeneration through affecting ESC regulation. Evidence has shown that ESC activity is regulated through ang-II receptor and therefore lead to epidermal regeneration in skin. Liao et al 48 performed an in vivo study on captopril treated male Wistar rats to determine the role of endogenous RAS in epidermal regeneration. They found that ACE is expressed by ESCs in the borders of wounded skin. This team, further demonstrated that ESCs in human foreskin have functionally active RAS constitutes, including ACE 1&2, Ang-II, and AT1R & AT2R. It was shown that the ACE-Ang-II pathway have a role in the maintenance of ESCs through AT1R & AT2R. The molecular mechanism of the role of RAS system in epidermal regeneration was additionally identified. Extracellular signal-Regulated Kinase (ERK) and signal transducers and activators of transcription STAT1 and STAT3 were targeted by Ang-II which was modulated by the AT2R and AT2R. ERK has been demonstrated to be involved in directional migration of fibroblasts in wounded area. Moreover, STAT1 and STAT3 signaling pathways are involved in wound healing through keratinocyte migration 23,24.
Regenerative role of renin angiotensin in wound healing: Wound healing is a complex and highly regulated physiological process aimed at restoring tissue integrity and function after injury. It involves a well-orchestrated sequence of events, including hemostasis, inflammation, proliferation, and tissue remodeling. Hemostasis involves blood clot formation to minimize blood loss, while inflammation is characterized by immune cell recruitment and removal of debris. During proliferation, new tissue is generated, and angiogenesis occurs to supply nutrients and oxygen. Finally, tissue remodeling leads to the restoration of tissue strength and function 49.
RAS activation is involved in various cellular pathways which can lead to wound healing 15. A damaged skin stimulates inflammatory cytokines and oxidative stress through angiotensin receptors. RAS is involved in blood coagulation in wound site. Additionally, it can accelerate fibroblast/myofibroblast and keratinocyte proliferation and migration. Secretion of extracellular fluid proteins is the last mechanism in which RAS can help skin tissue regeneration after injury 15. It has been demonstrated that RAS receptors, AT1R & AT2R, show up at different times during wound healing. Type 1 receptor is increased in inflammatory phase and type 2 is upregulated in proliferation phase. This mechanism indicates that the type 1 receptors enhance the re-epithelialization, and type 2 modulates granulation tissue formation. These data show that the expression of each component in RAS is depended on the phase of wound healing 33.
Studies which investigate the relationship between RAS and wound healing are mainly based on the inhibition of angiotensin receptors to observe the macroscopic and microscopic reactions of the skin. Studies on the knockout mice showed that when RAS is inhibited by receptor antagonists, the wound healing process was affected. This process was a combination of reduced epithelialization, wound construction, and angiogenesis 50.
Chronic wounds, including diabetic ulcers, affect people with metabolic diseases around the world. These wounds result from a combination of factors, including peripheral neuropathy, peripheral arterial disease, and impaired immune response 51. Microvascular events occurring as a result of diabetes can affect skin circulation. About 30% of the patients with diabetes deal with skin complications 20. ARBs are class of medications commonly used to treat conditions like high blood pressure and heart failure. They work by blocking the effects of Ang-II, a hormone that can narrow blood vessels and raise blood pressure, ultimately helping to relax blood vessels and lower blood pressure 52. Studies have shown that RAS could affect the pathogenesis of diabetic wound repair. Kamber and colleagues 53 conducted a research to investigate the effect of Losartan, an ARB, on wound healing in streptozotocin induced C57BL/6J diabetic male mice. The results of their study demonstrated that the daily use of Losartan (10 mg/kg through oral gavage) facilitated the healing process through the migration of keratinocytes from epidermis. Also, the intervention group showed significantly lesser open wounds than control group. The investigations of α-SMA demonstrated that myofibroblast contraction was higher in intervention group, indicating that Losartan can enhance wound healing through myofibroblast contraction. In this study, tissue vascularity in the stroma of wounded skin was lower in diabetic mice.
Similarly, in a clinical study reported by Lapray and coworkers 54 conducted a study on 276 diabetic patients with diabetic foot ulcers who were treated with ARBs, it was demonstrated that patients treated with angiotensin receptor inhibitors had a higher rate of wound healing than those not treated.
The expression of RAS components in burn injury was investigated by Jadhav et al in 2012 55. They found that after a burn injury (with silicone mold immersed in 75°C exposed to body surface for ten seconds) in mice, the RAS components, including AT1R & AT2R in keratinocytes and fibroblasts were decreased. It was demonstrated that this reduction is associated with a downregulation in collagen deposition and keratinocyte migration within the wounded site, and as a result, the re-epithelialization process would be affected.
The role of renin angiotensin system in scar formation and tissue remolding: Scar formation and remodeling constitute a complex and dynamic process that occurs in response to tissue injury. The mechanism involves several sequential phases. The initial inflammatory phase is triggered when an injury occurs, leading to blood clot formation, debris removal, and inflammation, primarily driven by white blood cells 56. Subsequently, during the proliferative phase, fibroblasts migrate to the wound site and produce collagen, creating a provisional extracellular matrix to bridge the wound. In the remodeling phase, collagen fibers are reorganized and cross-linked over a period of weeks to months, mediated by Matrix Metalloproteinases (MMPs) and Tissue Inhibitors of Metalloproteinases (TIMPs) 57. Finally, in the scar maturation phase, the collagen continues to remodel and mature, resulting in a scar that often becomes less conspicuous as the collagen fibers align and vascularity decreases 58. Transforming Growth Factor-beta 1 (TGF-β1) also plays a central role in scar formation by stimulating fibroblast proliferation and promoting the synthesis of extracellular matrix components, including collagen. This cytokine is essential for wound healing, as it helps in tissue repair and the closure of wounds 22,59. However, imbalances in TGF-β1 activity, with excessive or prolonged signaling, can lead to the overproduction of collagen and fibrosis, which contributes to the formation of hypertrophic or keloid scars. Thus, the regulation of TGF-β1 is critical in determining the quality and appearance of scar tissue, influencing whether the scar is inconspicuous or more pronounced 59.
Blood channel physiology abnormalities of many types contribute to scar formation. During wound healing, a dysfunction in vascular tissue, such as increased vascular permeability and density, accelerates the inflammatory phase, leading to exaggerated fibroblast activity and, also, the production of hypertrophic scars 60. These two disorders have a more significant number of vessels than normal skin 61, and all successful therapies inhibit aberrant vascularity, at least in part 61. AT1R is usually expressed in endothelial and muscle cells by the skin's blood vessels. Kurosaka et al discovered that the expression of Vascular Endothelial Growth Factor (VEGF) mRNA is reduced in AT1R knockout (AT1Ra-/-) mice or in animals that were administered AT1 receptor antagonists (ARBs) 50. In addition, oral treatment with candesartan inhibits angiogenesis in rats during wound healing 62.
In damaged human skin, the expression of AT1R and AT2R receptors is enhanced 63. Morihara et al showed increased ACE activity in human-damaged skin relative to normal skin, with the maximum activity detected in scar tissue 64. The number of AT1R in adult scars is much higher than in normal skin 65. In another study, researchers discovered that the concentration of Ang-II in skin samples separated from keloid patients was substantially more significant than that of normal skin. Compared to normal skin and hypertrophic scars, the expression of AT1R receptors was higher in keloid tissue 66.
Hedayatyanfard and colleagues also stablished a study to determine the anti-scar formation properties of Losartan. They found that topical application of Losartan potassium (5%) ointment on hypertrophic keloid and scar twice could effectively reduce keloid and scar formation 67. Since the scar formation in the surgical wound after thyroidectomy is an essential factor in aesthetic appearance of a patient, so it is important to prevent unaesthetic scars. Hu et al 68 indicated that administration of both ACE inhibitors and ARBs in patients postoperatively, could decrease scar formation. However, the formulation used in this study is not topical which is inconsistent to Hedayatyanfard’s study. In another assessment, it was shown that in AT1R deficient mice, the collagen content was reduced, the adipose tissue was replaced in wound scar, and TGFβ contents were locally increased. On contrary, in mice with A2TR deficiency, the wound healed faster; however, the inflammatory response was exaggerated through the pro-inflammatory effects of the type 1 receptor activity 69.
In other studies, to confirm the role of RAS in wound healing, ACE Inhibitors (ACEIs) were administered to rats. The rats showed a reduced scar formation due to the modulation of RAS activity. This means that although RAS is involved in wound healing by promoting stem cell proliferation and epithelialization, uncontrolled expression of RAS components can negatively impact the wound healing by scar formation 70. It was also indicated that scar formation could be more intense in rats with hypertension. So, the inhibition of hypertension with Captopril, can effectively reduce scar formation in hypertensive rats through inhibition of α-SMA, Ki67, and VEGF 71.
It was demonstrated that scar tissues from the treated mice with 0.2% Losartan urea cream or 0.1% Ramipril cream had significantly smaller scars compared to the negative control group. The proliferation of fibroblasts was less active and the collagen fibers were more regular in these groups. The efficacy of these treatments was similar to the positive control group, which received triamcinolone acetonide urea. The researchers also found that the effectiveness of the drugs did not solely depend on their ability to penetrate the skin. It was concluded that local application of ACEIs and ARBs can reduce scarring by decreasing the expression of collagen I, collagen III, p-Smad3, and TGF-β1. This reduction in scarring is a positive outcome, and it indicates that these treatments may be effective in promoting more favorable wound healing and scar remodeling 72.
ACE inhibitors have been shown to have antifibrogenic properties 73,74. In vitro and in vivo studies demonstrate that ACE inhibitors have anti-fibrotic characteristics via inhibiting TGF-β1/SMAD and TGF-1/TAK1 pathways 75. According to the reports, ACEIs such as Ramipril and Captopril diminish scar size by inhibiting TGF and PDGF expression, TAK1 and SMAD 2/3 phosphorylation, and fibroblast proliferation 75-77.
Ang-II contributes to the recruitment of infiltrating cells into tissues by direct stimulation of inflammatory cells or by regulating the production of adhesion molecules and chemokines by resident cells 78. This may help tissue healing by regulating cell proliferation and matrix creation. In addition, increased co-expression of TGF- β1 and Type 1, 3, and 4 collagen is reported in many scarring types, such as systemic sclerosis, post-burn hypertrophic scar, and keloids 79. It was also consistent with the findings of a study which utilized ARBs as topical agents to facilitate wound healing and reduce scar formation by regulation of collagens I, II, III, and Smad3, and TGF-β 72.
Discussion :
The present review aimed to discuss the molecular mechanisms of RAS in different phases of wound healing process (Table 1). However, this study focuses on the underlying molecular mechanisms of skin regeneration post-injury (Figure 1). Every step of wound healing can be affected by RAS and its components, including Ang-II, angiotensin receptors (AT1R and AT2R), and ACE. The evidence about the role of RAS components in wound healing is still controversial. It is mentioned that RAS components are locally expressed in the wounded skin. The primary role of RAS has been considered as blood pressure control, but with the existing literature about RAS expression in wounded skin, one of its most important roles in human physiology is revealed. Wound healing requires stabilized interaction between AT1R and AT2R activity. Therefore, before any intervention for pharmacotherapy, considering the maintenance between these two receptors is crucial.
Most of the studies indicate that the inhibition of RAS with ARBs (such as Losartan, Valsartan, etc.) and ACE inhibitors (Captopril, Enalapril, etc.) play a significant role in tissue remodeling in the last phase of wound healing. It has been shown that the inhibition of RAS, whether orally or topically, can inhibit scar formation and fibrosis through the downregulation of inflammatory and fibrogenic agents, such as TGF-β, VEGF, SMAD2/3, and TAK1, PDGF-BB, HSP47, etc. These data prove the hypothesis that "RAS can be a potential pharmacotherapy target for wound healing". Local administration of RAS regulators might lead to less scar formation and inflammation in the last phase of wound closure. It is obvious that identifying the exact mechanism of local administration of RAS modulators requires precise randomized controlled trials.
Conclusion :
Elucidating the precise local expression patterns of RAS components in wounded skin and the delicate equilibrium between AT1R and AT2R activities holds pivotal importance for the development of effective pharmacotherapeutic interventions. Notably, compelling evidence points to the potential benefits of RAS inhibition through ARBs and ACE inhibitors in guiding tissue remodeling and attenuating scar formation during the latter phases of wound closure. Future research endeavors should endeavor to unravel the nuances of local RAS regulator administration and its capacity to mitigate scar formation and inflammation. The imperative to undertake meticulous randomized controlled trials to decipher the exact mechanisms and therapeutic applications of RAS in the context of wound healing remains paramount. These explorations pave the way for innovative clinical interventions and enhanced scar management strategies.
Acknowledgement :
Figure was created with Biorender.com.
Conflict of Interest :
Authors declare that there is no conflict of interest.
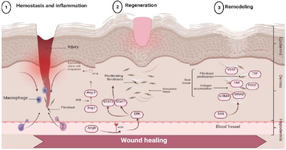
Figure 1. The impact of the renin-angiotensin system on the process of wound healing.
|
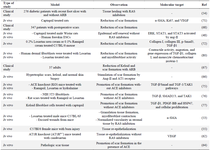
Table 1. The role of RAS components in different wound models and their target molecular mechanisms
* RAS: Renin Angiotensin System; Escs: Epidermal Stem cells; ARB: Angiotensin Receptor Blocker; ACE: Angiotensin Receptor Enzyme; PDGF: Platelet-Derived Growth Factor; HSP47: Heat Shock Protein 47.
|
|