Poultry Gastrointestinal-derived Lactic Acid Bacteria (pGIT-d-LAB) Inhibit Multiple Antibiotics Resistance Bacterial and Fungal Pathogens
-
 Adeniyi, Bolanle
Department of Pharmaceutical Microbiology, Faculty of Pharmacy, University of Ibadan, Ibadan, Nigeria, Tel: + +23 48023451480; E-mail: baaadeniyi@yahoo.co.uk
Adeniyi, Bolanle
Department of Pharmaceutical Microbiology, Faculty of Pharmacy, University of Ibadan, Ibadan, Nigeria, Tel: + +23 48023451480; E-mail: baaadeniyi@yahoo.co.uk
-
Adesuyi , Abimbola
-
Department of Pharmaceutical Microbiology, Faculty of Pharmacy, University of Ibadan, Ibadan, Nigeria
-
Ayeni , Funmilola
-
Department of Pharmaceutical Microbiology, Faculty of Pharmacy, University of Ibadan, Ibadan, Nigeria
-
Ogunbanwo , Temitope
-
Department of Microbiology, Faculty of Science, University of Ibadan, Ibadan, Nigeria
-
Agidigbi, Taiwo
-
Department of Pharmaceutical Microbiology, Faculty of Pharmacy, University of Ibadan, Ibadan, Nigeria
Abstract: Background: To develop a probiotic formulation for poultry feed, a few poultry gastrointestinal derived lactic acid bacteria (pGIT-d-LAB) were isolated from chicken intestinal specimens and in vitro experiment was performed to evaluate their efficacy as potential probiotic candidate.
Methods: A total of 6 strains of LAB: Lactobacillus brevis (L. brevis), Lactobacillus acidophilus (L. acidophilus), Lactobacillus casei (L. casei), Pediococci spp., Lactobacillus fermentum (L. fermentum) and Lactobacillus plantarum (L. plantarum) were isolated and cultured for collection of Cell Free Supernatant (CFS). CFS collected was tested against pathogenic bacterial isolated from chicken feces as well as prevalent fungal pathogens, utilizing agar-well diffusion techniques. A preliminary investigation into the susceptibility of the pathogens to diverse antibiotics and antifungal drugs was conducted. Bacterial pathogens exhibiting resistance to a minimum of three classes of antibiotics were subsequently identified for pGIT-d-LAB CFS screening.
Results: The observed results revealed that the CFS derived from the isolates exhibited varying degrees of growth inhibition against different pathogens. Among the tested pGIT-d-LAB isolates, L. acidophilus demonstrated the most prominent zone of inhibition, measuring 18 mm against Klebsiella pneumoniae ZTAC 1233. Notably, Citrobacter diversus ZTAC 1255 showed resistance to all tested pGIT-d-LAB. Quantification of the metabolites produced was performed, and peak production levels was determined. L. acidophilus produced the highest amount of lactic acid (1.789g/l), Pediococci spp. produced the highest amount of diacetyl and H202 (1.918g/l) (0.0025g/l) at 48 hr peak values respectively.
Conclusion: The test isolates are potential probiotic candidates for controlling pathogens in poultry.
Introduction :
Lactic Acid Bacteria (LAB) are a group of bacteria that produce lactic acid as a byproduct of their metabolism. They are Gram-positive, non-spore-forming bacteria and are commonly found in various environments, including plants, animals, fermented food products and the human gastrointestinal tract 1,2. Their antimicrobial properties, ability to modulate the immune system, and capacity to inhibit the growth of pathogens make them promising candidates for managing infections 1-3. Some of the most well-known genera of lactic acid bacteria include Lactobacillus, Propionibacterium, Streptococcus, Bifidobacterium, Pediococcus, Leuconostoc, and Lactococcus 1-3. These genera encompass numerous species and strains that have different characteristics and are employed in various industrial and commercial applications 4-6. They are generally considered safe for human consumption and are often referred to as probiotics due to their potential health benefits 6,7-9.
Probiotics are live micro-organisms that confer health benefits when consumed in adequate amounts. LAB can help maintain a healthy balance of micro-organisms in the gut and contribute to digestion, nutrient absorption, and immune function 9-12. Additionally, LAB have antimicrobial properties that can inhibit the growth of harmful bacteria, making them useful in food preservation and the prevention of foodborne illnesses 6,9,13-16. Their diverse metabolic capabilities and beneficial properties make them a significant group of bacteria in both scientific and industrial contexts 17,18.
In the context of bacterial infections, LAB exerts their antimicrobial effects through multiple mechanisms. LAB can produce organic acids such as lactic acid and acetic acid, which lower the pH of their environment, creating an inhospitable environment for many pathogenic bacteria 9,11,12,19. LAB can produce bacteriocins, which are antimicrobial peptides that selectively target and inhibit the growth of other bacteria. These mechanisms contribute to the competitive exclusion of pathogens and the maintenance of a balanced microbial ecosystem 20-23.
Furthermore, LAB's role in managing fungal infections has also been explored 24-29. Certain strains of LAB have demonstrated the ability to inhibit the growth of various fungal pathogens through competitive exclusion, production of antifungal compounds, and modulation of the host's immune response 29,30. LAB's potential to counter fungal infections is particularly significant, given the rising concern of antifungal resistance and the limited treatment options available. Probiotics have demonstrated encouraging potential in both the prevention and treatment of allergic conditions, encompassing disorders like atopic dermatitis and allergic rhinitis 31,32. Probiotics hold clinical significance in the realm of gastrointestinal disorders, spanning conditions such as irritable bowel syndrome, diarrhea, inflammatory bowel disease, and antibiotic-associated diarrhea 31-36. Metabolic disorders have attained a widespread epidemic status on a global scale, search for probiotics for management of disease including but not limited to obesity, diabetes, and non-alcoholic fatty liver disease is on increased 30-39.
The primary objective of this study was to isolate, characterize, and evaluate pGIT-d-LAB strains possessing probiotic attributes from poultry chicken. These strains were intended for potential use as a supplements or alternative to antibiotics in the context of poultry infection control and management.
Materials and Methods :
Poultry sample collection and preparation: Thirty 30 intestinal fecal samples were collected randomly from broilers in a commercial poultry farm in Ibadan, southwestern Nigeria for the isolation of poultry gastrointestinal-derived lactic acid bacteria (pGIT-d-LAB). Sample bottles and test tubes containing 5 ml sterile peptone water were labeled accordingly after transporting samples into the laboratory. The workbench was disinfected by cleaning with 70% ethanol, and a loopful of each sample was then added into 5 ml of sterile peptone water and incubated aerobically at 37°C for 24 hr.
Isolation, identification, and characterization: Serial dilutions of the cultures from the peptone water were then performed according to method described with slight modification 13,40. Pour plate method was used for the isolation of the isolates by transferring 0.1 ml of selected dilution aseptically into the sterile plates containing de Man Rogosa Sharpe agar (for isolation of LAB) and various media were used to isolate bacterial species. The plates were incubated micro-aerobically at 37°C (Oxoid: Campygen, UK) after which pure colonies were isolated. Identification and characterization of isolates were carried out according to conventional microbiological standard practices (macroscopic, microscopic, physiological, and biochemical tests).
Antibiotic susceptibility test: Antibiotic susceptibility test for each bacterial pathogen was performed using the disc diffusion method. 0.1 ml of actively growing bacterial culture containing approximately 1×106 cfu/ml compared to McFarland standard was inoculated into Mueller Hinton agar by pour plate method. Different antibiotic sensitivity- discs such as Nitrofurantoin 300 µg, Nalidixic acid 30 µg, Ofloxacin 30 µg, Augmentin 30 µg, Tetracycline 30 µg, Chloramphenicol 30 µg, Amoxicillin 25 µg, Gentamicin 10 µg, Clotrimazole 5 µg, Erythromycin 5 µg and Cloxacillin 5 µg were placed on the solidified agar surface. For fungal isolates, itraconazole and Griseofulvin were used as positive control. The plates were incubated aerobically at 37°C for 24 hr. After 24 hr, the diameter of the zone of inhibition of each disc was measured. The zone of inhibition corresponds to the antibiotic activity of each disc. The relative susceptibility of each isolate to each antibiotic was shown by a clear zone of inhibition resistance was defined by the absence of a zone of inhibition or inhibition less than 10 mm in diameter.
Determination of in vitro antimicrobial activity: Cell Free Supernatants (CFS) of pGIT-d-LAB isolates were obtained from MRS broth cultures after 72 hr incubation at 30°C by centrifugation at 10,000×g for 10 min at 4°C. The inhibitory activities of the CFS against the pathogenic bacteria and fungal were assayed by the agar well diffusion tests. The plates were incubated at 37°C for 24 hr after which clear zones were examined around the wells. The antimicrobial effects were recorded by measuring the zone of inhibition around the well.
Quantitative determination of antimicrobial compounds produced by pGIT-d-LAB: For this measurement, the test organisms were grown in MRS broth for 72 hr and samples collected at 12 hr interval up to 72 hr and centrifuged at 3,000×g for 15 min to obtain supernatant.
Lactic acid: Lactic acid production was determined slight modification to the method described 41 by titrating 25 ml of the supernatant fluid of the test organism and adding three drops of phenolphthalein as indicator. 0.1 M NaOH was slowly added from a burette onto the samples until a pink colour appeared. Each ml of 0.1 M NaOH is equivalent to 90.08 mg of lactic acid.
Hydrogen peroxide (H2O2): Twenty (20) ml of 0.1 M diluted tetraoxosulphate (VI) acid was added to 25 ml of the supernatant fluid of the test organism. Titration was carried out with 0.1 M potassium permanganate (KMnO₄). Each ml of 0.1 M potassium permanganate is equivalent to 1.79 mg of H2O2 solution. Decolourization of the sample was regarded as the end point. The volume of H2O2 produced was then calculated 42.
Diacetyl: This was determined by transferring 25 ml of the supernatant fluid of the test organism into 100 ml flask and 7.5 ml of hydroxylamine solution was used for the residual titration. The flasks were titrated with 0.1 M HCL to a greenish yellow end point using bromophenol red as indicator. The equivalent factor of HCL to diacetyl is 21.5 mg. The concentration of diacetyl produced was calculated according to the method of food chemical codex 43.
Data analysis: All data were replicated and expressed as means ± SEM (standard error of the mean) using student t-test one-way ANOVA (analysis of variance) on Microsoft excel sheet. Graphical representation was performed using software GraphPad Prism9 (GraphPad software Inc., San Diego, USA).
Results :
The occurrence of common pathogenic bacterial spp. in poultry feces: A total of 25 pathogenic bacteria were isolated from the fecal samples of chicken (Figure 1). The isolates were subjected to standard microbiological and biochemical tests to identify the bacteria species.
Among the 25 microorganisms identified, the distribution is as follows: Escherichia coli (E. coli) (5), Pseudomonas spp. (5), Klebsiella pneumonia (3), Bacillus subtilis (B. subtilis) (3), Micrococcus virians (M. virians) (3), Mycobacterium spp. (2), Salmonella typhi (2), Citrobacter freundi (1) and Citrobacter diversus (C. diversus) (1) (Figure 1A). In overall, result of the prevalence of bacteria pathogens in the feces of chicken shows higher percentage of prevalence with E. coli and Pseudomonas spp. (20%) followed by Klebsiella and Bacillus (12%), Mycobacterium spp. and Lactobacillus delbrueckii (8%), while the genus Citrobacter spp. (4%) showed the lowest prevalence (Figure 1B).
Occurrence of LAB spp. in poultry intestinal feces: Following standard procedures for isolation and identification, a total of 30 strains of LAB were isolated from chicken intestine. The isolates were identified as Lactobacillus brevis (L. brevis) 14, Lactobacillus plantarum (L. plantarum) 7, Lactobacillus acidophilus (L. acidophilus) 5, Pediococci spp. 2, Lactobacillus casei (L. casei) 1, and Lactobacillus fermentum (L. fermentum) 1 (Figure 2A). The percentage occurrence of pGIT-d-LAB from the chicken intestine was found to be L. brevis (46.66%), followed by L. plantarum (23.33%), and L. acidophilus (16.66%), while L. casei and L. fermentum both have (3.33%), respectively. The varying percentages of occurrence of pGIT-d-LAB isolates indicate a wide distribution of LAB strains in the intestines of chicken (Figure 2B).
Antibiogram assay: The sensitivity of the different pathogenic bacteria to commercial standard available antibiotics sensitivity disc containing known concentration of antibiotics was investigated. All Gram-negative bacteria showed susceptibility to chloramphenicol except M. vivian’s ZTCC 125. Also, all Gram-positive bacteria showed susceptibility to clotrimazole except Bacillus subtilis ZTAC 1247, Mycobacterium spp. ZTCC 1250, and M. vivians ZTCC 1251. All the pathogenic bacteria demonstrated resistance to Amoxicillin, Augmentin, and Tetracycline, Erythromycin and Cloxacillin. All the Gram-negative bacteria showed resistance to Nalidixic acid except E. coli ZTCC 1241 and E. coli ZTCC 1242 which showed intermediate susceptibility. All Gram-positive bacteria demonstrated resistance to Amoxicillin, Erythromycin, Cloxacillin, Augmentin and Tetracycline. In general, all the bacteria pathogens demonstrated multiple drug residence as defined as resistance to at least two different classes of antibiotics (Table 1). The use of antimicrobial agents such as antibiotics as a preventive or control measure of poultry diseases has given rise to the extensive evolution of antimicrobial resistance among pathogenic bacteria. Most of these pathogens might also be Extended Spectrum Beta–Lactamase (ESBL) producers which are global health concerns and major problems for the treatment of different infections caused by Enterobacteriaceae 44,45.
pG IT-d-LAB exhibit antagonist action against multiple antibiotics resistance bacterial: The CFS of the pGIT-d-LAB isolates produced antagonistic activities against all the pathogenic bacteria in this study except Citrobacter diversus ZTAC 1255. The widest zone of inhibition of 18 mm in diameter was produced by L. acidophilus against Klebsiella pneumoniae (K. pneumoniae) ZTAC 1233 and L. casei against Citrobacter freudi ZTAC 1249. The lowest zone of inhibition of 9 mm in diameter was produced by L. plantarum against Pseudomonas spp. ZTAC 1252. The CFS of L. brevis, L. acidophilus and L. casei also showed inhibitory activity against K. pneumoniae ZTAC 1233. Metabolites produced by the pGIT-d-LAB isolates showed an impressive antagonistic activity against Mycobacterium spp. ZTAC 1236, M. virians ZTAC 1235. In addition, it can be observed from the table that none of CFS of the isolates showed activity against C. diversus ZTAC 1255 (Table 2).
CFS of pGIT-d-LAB exhibit antagonist action against fungal pathogens: In this study, the CFS of the pGIT-d-LAB isolates produced antagonistic activity against all the selected fungal pathogens except Candida valida (Candida valida) ZTAC 1409 (Table 3). The largest zone of inhibition (24 mm) was produced by L. acidophilus against Candida albicans (C. albicans) ZTAC 1401. The CFS of the isolate of L. brevis was exceptional as it was the only isolate that was able to exhibit antagonistic activity against Candida tropicalis (C. tropicalis) ZTAC 1403 and Epidermophyton rubrum (E. rubrum) ZTAC 1411. The CFS of L. casei was unable to produce any antagonistic activity against the fungal pathogens. Trichophyton rubrum (T. rubrum) ZTAC 1407 was the most susceptible dermatophyte to metabolites produced by pGIT-d-LAB isolates. The largest zone of inhibition (17 mm) against the dermatophytes was shown by Pediococci against T. rubrum ZTAC 1407. Candida krusei ZTAC 1405 was inhibited by the CFS of L. plantarum (18 mm) and Pediococci spp. (21 mm). The CFS of L. fermentum did not inhibit C. tropicalis ZTAC 1403, C. albicans ZTAC 1401, C. krusei ZTAC 1405 and C. valida ZTAC 1409 (Table 3). In contrast to our finding, Ronnqvist et al, 2007 46 reported that a LAB isolate, L. fermentum Ess-1, showed activity against C. albicans and Candida glabrata. The antagonistic activity of LAB metabolites on the two dermatophytes; T. rubrum ZTAC 1407 and E. rubrum ZTAC 1411 showed that both were sensitive to the antimicrobial producing LAB to varying degrees except that E. rubrum ZTAC 1411 showed susceptibility to the metabolite produced by L. brevis only. T. rubrum strain ZTAC 1407 displayed the greatest susceptibility among the dermatophytes examined in this research. Among the tested strains, Pediococcus spp. exhibited the most potent activity (17 mm) against T. rubrum ZTAC 1407. Interestingly, only the CFS generated by L. casei showed no impact on the growth of the dermatophyte T. rubrum ZTAC 1407. Beyond directly inhibiting fungal growth, LAB also possess the capability to selectively hinder the production of mycotoxins and immobilize mycotoxins by attaching to their surface. The antimicrobial capacity of LAB has been well documented 6. In general, Lactobacillus species were the most inhibitory to test organisms followed by Pediococci spp. and Leuconostoc spp. (Table 3).
Quantification of metabolites produced by pGIT-d-LAB" Quantitative investigation of three different antimicrobial compounds produced by pGIT-d-LAB species was investigated. We found that metabolites production and concentration varied across LAB species (Figures 3-5). In this study, Pediococci spp. produced the highest concentration of diacetyl (1.918 g/l) while the lowest concentration was produced by L. casei (1.382 g/l) (Figure 3A). The heatmap showed that all the isolates produce the highest concentration of metabolites at 48 hr peak; L. brevis 1.701 g/l, L. plantarum 1.621 g/l, L. acidophilus 1.704 g/l, Pediococci spp. 1.918 g/l, L. casei 1.382 g/l and L. fermentum 1.617 g/l, respectively (Figure 3B).
Among the top producers of lactic acid at 24, 48 and 72 hr, L. plantarum, produced 1.644 g/l, 1.707 g/l, 1.482 g/l, L. acidophilus produced 1.534 g/l, 1.789 g/l and 1.428 g/l while L. fermentum produces 1.535 g/l, 1.715 g/l and 1.628 g/l, respectively (Figure 4A). The production of lactic acid among these isolates was peak at 48 hr, L. acidophilus produced highest volume of 1.789 g/l while L. casei produced the lowest volume of 1.164 g/l (Figure 4B). H2O2 production was also determined among the pGIT-d-LAB isolates. Overall, the concentration of H2O2 produced were generally low among all pGIT-d-LAB isolates (Figure 5A). The highest yield was produced by Pediococci spp. (0.0025 g/l) and the lowest yield was produced by L. casei (0.0014 g/l) after 48 hr incubation (Figure 5B).
Discussion :
The continuous alternate search for antibiotics due to development of drug resistance offers a distinct advantage to explore novel probiotic strains. Consequently, the necessity to cultivate host-specific probiotics to attain optimal health advantages and enhance livestock performance becomes paramount. This study aimed to evaluate the prophylactic potential of a LAB from poultry against common pathogenic organisms. The application of probiotics in the poultry industry as suitable alternative to antibiotics performance has garnered significant interest in contemporary times. Beyond the myriad advantageous attributes of probiotics, the extraction of probiotic strains from their indigenous hosts stands as the foremost choice. Microbial strains sourced from their natural habitat possess an inherent familiarity with the gastrointestinal tract, thereby enabling them to naturally propagate and more effectively manifest the desired advantageous outcomes, surpassing strains obtained from alternative sources. It was interesting to note that none of the antimicrobials produced by pGIT-d-LAB isolates showed inhibitory activity against the organism producing it.
According to our findings, we concluded that the rise in lactic acid production over time is attributed to a decrease in pH, facilitating the growth of LAB at the expense of other competing organisms. This is in conformity with reports from other studies 6,9. The antimicrobial impact of lactic acid arises from its non-ionized state, which facilitates its penetration through membranes. Subsequently, within the cytoplasm, it releases hydrogen ions, thereby impeding essential cellular functions which will eventually lead to death. Additionally, we believe that the efficacy and action of pGIT-d-LAB against pathogenic micro-organisms in this study was based on the action of H2O2, lactic acid, bacteriocins, diacetyl and a combination of various uncharacterized antimicrobial molecules released into the cellular milieu of the offending microorganisms. Our observational study aligns with the findings reported by other researchers 17,20,38. However, the CFS of L. fermentum did not demonstrate inhibitory effects on Candida spp. examined. This result sharply contrasts with the findings of Ronnqvist et al 46, who reported the inhibitory activities of Lactobacillus spp. against Candida spp. Although showing great potential, the clinical utilization of LAB of poultry origin as probiotics in infection treatment demands more in-depth research, comprehensive clinical trials, and established protocols to substantiate their effectiveness and safety. These guidelines underscore the importance of meticulously planned investigations that can substantiate the efficiency and safety of LAB strains in managing infections. We understand that diverse strains of LAB exhibit distinct antimicrobial attributes, this has underscored the significance of discerning strains with superior efficacy against bacterial and fungal pathogens.
Conclusion :
In conclusion, pGIT-d-LAB exhibit considerable potential as viable probiotics for addressing bacterial and fungal infections in poultry. Their diverse array of mechanisms, encompassing the synthesis of antimicrobial agents such as lactic acid, H2O2 and immunomodulatory effects, positions them as compelling contenders for the management of microbial infections. Nevertheless, additional research is imperative to ascertain the most suitable strains, dosages, and delivery strategies, while also adhering to regulatory standards. Only through these endeavors can widespread clinical implementation be responsibly pursued.
Conflict of Interest :
The authors declare that they have no competing interests.
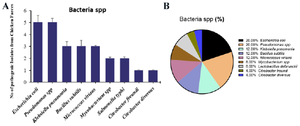
Figure 1. A) The occurrence and B) proportional percentage of pathogenic bacteria from chicken intestine.
|
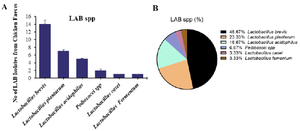
Figure 2. A) The occurrence and B) proportional percentage of pGIT-d-LAB isolated from intestine.
|
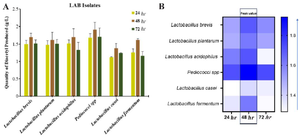
Figure 3. A) Quantity of Diacetyl produced by pGIT-d-LAB isolates, B) heatmap of the peak value.
|
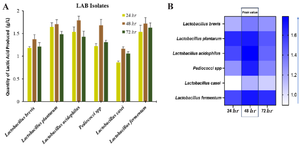
Figure 4. A) Quantity of Lactic Acid produced by pGIT-d-LAB isolates, B) heatmap of the peak value.
|
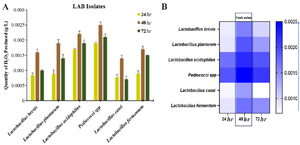
Figure 5. A) Quantity of H2O2 produced by pGIT-d-LAB isolates, B) heatmap of the peak value.
|
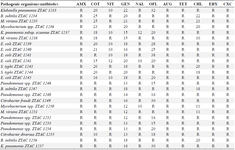
Table 1. Antibiogram Showing Drug Resistance and Sensitivity Patterns of Test Bacteria (zone of inhibition in mm)
Augmentin (30 mg), Tetracycline (30 mg), Nitrofurantoin (300 mg), Chloramphenicol (30 mg), Ofloxacin (30 mg), Nalidixic acid (30 mg), Amoxycillin (25 mg), Clotrimazole (25 mg), Gentamycin (10 mg), Cloxacillin (5 mg), and Erythromycin (5 mg). * Resistance (R) = <10 mm; **Sensitivity = ≥10 mm.
|
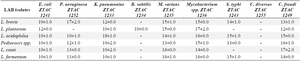
Table 2. Activity of LAB against pathogenic bacteria (diameter of zone of inhibition in mm)
* Diameter of the cork borer = 8 mm.
|
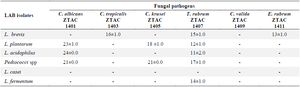
Table 3. Activity of LAB against pathogenic fungal (diameter of zone of inhibition in mm)
* Diameter of the cork borer = 8 mm.
|
|