A Simple High Yield Technique for Isolation of Wharton's Jelly-derived Mesenchymal Stem Cell
-
Niknam, Bahare
-
Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Azizsoltani , Arezou
-
Department of Medical Biotechnology, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences , Tabriz, Iran
-
Heidari, Neda
-
Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Tokhanbigli, Samaneh
-
Basic and Molecular Epidemiology of Gastrointestinal Disorders Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Alavifard, Helia
-
Basic and Molecular Epidemiology of Gastrointestinal Disorders Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Haji Valili, Mahsa
-
Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Amani, Davar
-
Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Asadzadeh Aghdaei, Hamid
-
Basic and Molecular Epidemiology of Gastrointestinal Disorders Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
 Hashemi , Seyed Mahmoud
Department of Immunology, Shahid Beheshti University of Medical Sciences, Tehran, Iran, E-mail: smmhashemi@sbmu.ac.ir
Hashemi , Seyed Mahmoud
Department of Immunology, Shahid Beheshti University of Medical Sciences, Tehran, Iran, E-mail: smmhashemi@sbmu.ac.ir
-
 Baghaei, Kaveh
Research Institute of Gastroenterology and Liver Disease (RIGLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran, E-mail: kavehbaghai@gmail.com
Baghaei, Kaveh
Research Institute of Gastroenterology and Liver Disease (RIGLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran, E-mail: kavehbaghai@gmail.com
Abstract: Background: The isolation of Mesenchymal Stem Cells (MSCs) from various tissues is possible, with the umbilical cord emerging as a competitive alternative to bone marrow. In order to fulfill the demands of cell therapy, it is essential to generate stem cells on a clinical scale while minimizing time, cost, and contamination. Here is a simple and effective protocol for isolating MSC from Wharton's Jelly (WJ-MSC) using the explant method with various supplements.
Methods: Utilizing the explant method, small fragments of Wharton's jelly from the human umbilical cord were cultured in a flask. The multipotency of the isolated cells, were confirmed by their differentiation ability to osteocyte and adipocyte. Additionally, the immunophenotyping of WJ-MSCs showed positive expression of CD73, CD90, and CD105, while remaining negative for hematopoietic markers CD34 and CD45, meeting the criteria for WJ-MSC identification. Following that, to evaluate cells' proliferative capacity, various supplements, including basic Fibroblast Growth Factor (bFGF), Non-Essential amino acids (NEA), and L-Glutamine (L-Gln) were added to either alpha-Minimal Essential Medium (α-MEM) or Dulbecco's Modified Eagle's Medium-F12 (DMEM-F12), as the basic culture media.
Results: WJ-MSCs isolated by the explant method were removed from the tissue after seven days and transferred to the culture medium. These cells differentiated into adipocyte and osteocyte lineages, expressing CD73, CD90, and CD105 positively and CD34 and CD45 negatively. The results revealed that addition of bFGF to α-MEM or DMEM-F12 media significantly increased the proliferation of MSCs when compared to the control group. However, there were no significant differences observed when NEA or L-Gln were added.
Conclusion: Although bFGF considerably enhances cell proliferation, our study demonstrates that MSCs can grow and expand when properly prepared Wharton's jelly tissues of the human umbilical cord.
Introduction :
Mesenchymal Stem Cells (MSCs) are multipotent cells capable of self-renewal and can differentiate into multiple lineages of connective tissue, such as osteocytes, adipocytes, and chondrocytes 1. These cells have gained significant attention for their minimal immunogenicity and immunomodulatory properties, making them valuable in the treatment of various disorders 2,3. Several clinical studies have shown the therapeutic effects of MSCs in various diseases such as COVID-19 4,5, liver cirrhosis 6, rheumatoid arthritis 7, heart failure 8, and Graft-Versus-Host Disease (GVHD) 9. To isolate MSCs, both adult and fetal/perinatal tissues can be utilized, including Bone Marrow (BM) 10, Adipose Tissue (AT) 11, the dental pulp 12, peripheral blood 13, placenta 14, amniotic membrane, placental decidua, umbilical cord 15 and Wharton's jelly 16,17. However, the limited proliferative capacity and low cell content of MSCs obtained from adult tissues, especially bone marrow, combined with the invasive and painful isolation procedures associated with an increased risk of infection have restricted their widespread application 18-21. Hence, finding alternative sources for clinical applications is needed.
Unlike adult MSCs, Wharton's Jelly of the human umbilical cord-derived MSCs (WJ-MSCs) have attracted great interest due to their numerous advantages, including unlimited availability, extraction of large quantities, affordability and higher proliferative capacity 16,22. The isolation of the umbilical cord is non-invasive and does not raise ethical concerns as it involves medical waste discarded at birth 23. Unlike, human Embryonic Stem Cells (ESCs), WJ-MSCs also produce larger quantities of anti-inflammatory cytokines and do not induce tumorigenesis 24. Consequently, treatment opportunities can be created from a redundant source.
WJ-MSCs are an attractive and appropriate source for cell therapies in allogeneic transplantation due to their ability to inhibit immunity and evade immune responses. They are almost devoid of MHC class II molecules and express low levels of MHC class I, CD80, CD86, and CD40 co-stimulatory molecules 25,26. Additionally, WJ-MSCs induce Treg cell proliferation, inhibiting effector cell responses to alloantigens 27. Unlike BM-MSCs, these cells produce higher levels of tolerogenic Transforming Growth Factor-β (TGF-β) and Interleukin (IL)-10 28. WJ-MSCs also contribute to immunosuppression by releasing soluble factors such as Hepatocyte Growth Factor (HGF), Prostaglandin E2 (PGE2), Indoleamine 2,3-dioxygenase (IDO), and HLA-G 16. Several studies have shown a relative increase in the expression of pluripotent markers in WJ-MSCs compared to other sources, suggesting that these cells are still in a more primitive state 29.
On the other hand, the expression of several immune molecules, including chemokines and immune regulators, is higher in WJ-MSCs than in other MSCs, such as BM-MSCs 30. These findings support the notion that WJ-MSCs are the more appropriate choice for processes such as wound healing. Considering the advancements in treatment methods and the progress of regenerative medicine, isolation, identification of specific features, as well as developing appropriate procedures for the large-scale proliferation of these cells could be crucial.
In this study, we describe step by step process of isolation and expanding cells using explant method, along with its modified variation. We examined differences in the proliferation capacity of isolated WJ-MScs, in the presence of various supplements, such as basic Fibroblast Growth Factor (bFGF), Non-Essential Amino acids (NEA), and L-Glutamine (L-Gln) in alpha-Minimal Essential Medium (α-MEM) and Dulbecco's Modified Eagle's Medium-F12 (DMEM-F12) basal culture media. The present study aimed to provide an easy, practical, and reliable method for harvesting a large number of WJ-MSCs with superior characteristics.
Materials and Methods :
Sample collection: After receiving approval and consent from the medical ethics committee (IR.SBMU.RIGLD.REC.1399. 062), Umbilical Cords (UCs) were collected from cesarean-section delivery under the aseptic condition at Royan Institute (Tehran-Iran). UCs were rinsed using Phosphate Buffer Saline (PBS) containing 1% Pen/ Strep-Amphotericin B (Gibco, Grand Island, NY) to remove surface blood. UCs were maintained in PBS supplemented with antibiotics, 100 U/ml penicillin-streptomycin (Gibco, Grand Island, NY, USA), stored at 4°C and transferred to the lab.
Explant culture: In explant protocol, after washing the umbilical cord with PBS, the vein and arteries were removed, and the UC was cut into small fragments (3-4 mm) and rinsed again with PBS until no further surface blood could be seen. The diced pieces were delivered into 25 cm2 flasks and incubated at 37°C under a 5% CO2 atmosphere in α-MEM (Gibco, Grand Island, NY, USA), supplemented with 15% Fetal Bovine Serum (FBS)-containing 200 U/ml Pen/Strep. The flasks remained unmoved for 7 days for MSCs to migrate from the tissue to adhere to the plastic. After that, half of the old medium was removed and replaced with the new culture medium. In the following, the growth of cells was monitored every day. The fragments were taken out after 10 days, and the adhering cells were allowed to grow. When the adherent cells reached 80% confluence (P0), they were trypsinized with 0.25% trypsin-EDTA (Gibco, Grand Island, NY, USA) and subcultured into new flasks. A cell culture microscope (Olympus, Japan) was used to evaluate the morphology of expanded MSCs.
Optimization medium for primary culture of the WJ-MSCs: We studied two different basal media and three supplements to find the optimum medium for the proliferation of WJ-MSCs. The 4th passage of cells with 80% confluency was detached using trypsin/EDTA 0.25% and was centrifuged at 450 g for 10 min. Cells were counted using trypan blue and reseeded at 30,000/cm2 density in 24 well plates and cultured in α-MEM media supplemented with 10% FBS. We designed experimental groups divided into two separated groups based on basal medium DMEM-F12/α-MEM containing 10% FBS and different combinations of supplements bFGF (10 ng/ml), NEA (1×), and L-glutamine (2 mM) as stated in a workflow (Figure 1) (all from Gibco, Grand Island, NY, USA). The seeded cells were treated with media groups. Cells were trypsinized and counted after exposure to the supplement for 48 and 96 hr.
Characterization of WJ-MSCs: Differentiation analysis of WJ-MSCs: P4 cells were used to investigate the WJ-MSCs' differentiation potential. The cells were seeded at 10,000 cells/cm2 in 12-well plates. After reaching approximately 70% confluence, a specific induction medium was added to each well. For the osteogenesis assay, the cells were grown in an osteogenic induction medium (Sigma-Aldrich, USA) that contained DMEM, 10% FBS, 10 nM Dexamethasone, 0.2 mM ascorbic acid 2-phosphate, and 10 mM glycerol phosphate. Two times a week for 21 days, the medium was changed. The induction media was withdrawn after the cells had been exposed to it for 21 days. Then, the cells were washed with PBS, fixed with 4% paraformaldehyde for 20 min at 4°C, and stained with 2% alizarin red at pH=7.2. For adipogenesis induction, the cells were cultured in an adipogenic medium (Sigma-Aldrich, USA) that contained DMEM enriched with 10% FBS, indomethacin (50 µg/ml) dexamethasone (1 µM), insulin (5 µg/ml), and isobutylmethylxanthine (0.5 mM).
The culture medium was changed every 3~4 days for up to 21 days. In the following, the cells were fixed in 4% paraformaldehyde and visualized with Oil Red staining (Sigma-Aldrich, USA). An inverted microscope (Olympus, Japan) was used to observe calcium deposits and lipid droplets.
Flow cytometry analysis: The surface markers of isolated cells were analyzed using Phycoerythrin (PE)-conjugated antibodies, including CD34, CD45, CD73, CD90, and CD105 (eBioscence, Germany). The cells were detached by trypsin/EDTA; after which they were exposed to particular antibodies. Briefly, the detached cells were incubated with each antibody in 100 µl staining buffer containing 2% FBS/PBS for 20 min at 4°C in dark conditions. Then, the cells were washed with a staining buffer to eliminate the unattached antibodies. Finally, the cells were suspended in a staining buffer and analyzed using a flow cytometry instrument (BD FACS flow cytometry, San Joes, CA, USA). Data analysis was performed using Flowing Software 2.5.1.
Statistical analysis: Data are presented as mean±SD. A t-test or one-way ANOVA analyzed comparisons between two or more than two groups with Tukey's post hoc test. **** p<0.0001, ***p<0.001, **p<0.01 and * p<0.05 was considered statistically significant. For the statistical tests, GraphPad Prism (version 8) was used.
Results :
WJ-MSCs culture isolation characterization: During the explant culture, by changing media in 7, 10, and 14 days post-WJ-MSCs isolation procedure, only adherent WJ-MSCs remained attached to the flasks, and most of the non-adherent cells were removed (Figure 2). The remaining WJ-MSCs cells were verified within different steps according to their morphology, surface marker expression, and ability to differentiate to another cell lineage. As shown in figure 2F, the isolated cells created a monolayer of spindle-shaped fibroblastic-like appearance.
WJ-MSCs differentiation potency: Human WJ-MSCs are spindle-shaped, fibroblast-like cells (Figure 3A). The isolated WJ-MSCs capability for multi-lineage formation was confirmed by osteoblast-like and adipocyte-like differentiation assay. Osteogenesis verified by alizarin staining showed the formation of calcium oxalates in differentiated WJ-MSCs (Figure 3B). In addition, applying oil-red O demonstrated WJ-MSCs differentiation to adipogenic-like cells (Figure 3C). On the other hand, no similar events were detectable in undifferentiated WJ-MSCs. These observations provide evidence for the multipotency of isolated and cultured WJ-MSCs.
WJ-MSCs cell surface markers expression: The minimum criteria for WJ-MSCs immunophenotyping are positive for CD73, CD90, and CD105, plus remaining negative for hematopoietic markers, including CD34 and CD45. As represented in figure 4, our isolated WJ-MSCs met the mentioned positive and negative selection criteria.
Different medium effects on the proliferation ability of isolated WJ-MSCs: WJ-MSCs were cultured in two distinct basal media, α-MEM or DMEM-F12, with the addition of supplements. The experimental groups were divided into three different groups: one with only L-Gln, another with NEA and L-Gln (NEA+ L-Gln), and a third group with bFGF added to the latter compound (bFGF+NEA+ L-Gln).
The proliferation of WJ-MSCs has been evaluated 48 and 96 hr post-treatment. Generally, there was no significant difference in cell proliferation rate between the same groups in both basic culture media. In detail, after 48 hr, the expansion of the cells was dramatically upper (~2 fold) in bFGF+NEA+L-Gln against the control group (p˂0.001). It has also shown better outcomes in comparison to L-Gln and NEA+L-Gln in both α-MEM and DMEM-F12 basal media. The expansion rate of cells in each treatment and time point is exhibited in figure 5. Similar results were obtained in 96 hr. However, L-Gln can increase the effectiveness of F12 in MSC expansion at 96 hr. The best expansion rate was seen in bFGF+NEA+L-Gln treatment of α-MEM and DMEM-F12 after 96 hr compared to the control group (~3 fold, p˂0.0001). In brief, for optimum expansion of WJ-MSCs, applying bFGF+NEA+L-Gln in both α-MEM and DMEM-F12 condition media is preferable.
Discussion :
MSCs are isolated from different tissues and characterized specifically. Although Bone Marrow-derived MSCs (BM-MSCs) have been extensively studied, stem cells from various embryonic tissues have recently attracted great attention due to their abundant availability and rich source of MSCs 31. One of these tissues is human Umbilical cord-derived Wharton's jelly. Although MSCs derived from Wharton's jelly have similar characteristics to those derived from BM-MSC, they have some advantages: a higher frequency and proliferation potential, differentiation into several cell lineages, and resistance to age-related changes 32-34. Due to their immunologically privileged status, allogeneic therapy can be conducted with MSCs 35. In this study, we aim to describe a naïve method of isolating WJ-MSCs for approximately 7 days. This method can shorten the primary culture time and increase the proliferation rate and the number of cells compared to the other methods, and reduces biological contaminations and costs 36. For successful MSC transplantation, it is crucial to prepare MSCs with high therapeutic potential. Developing a method for accelerating the proliferation of these cells is necessary for obtaining sufficient numbers of transplantable cells.
This study compared the ability of DMEM-F12 and α-MEM with different supplements, including bFGF, NEA, or L-Gln, to expand WJ-MSCs. Both DMEM-F12 and α-MEM contain essential and non-essential amino acids, which support proliferation of MSCs and preserves their characteristics. α-MEM has lower glucose level compared to DMEM-F12 as high glucose levels appear to adversely affect cultured stem cells through the oxidative stress pathway 37.
Decreased glucose concentration reduces autophagy and apoptosis while increasing colony size in the Colony Forming Unit (CFU) assay and MSC proliferation rate 38,39. Conversely, high glucose concentration, promotes the differentiation of MSC into osteocytes, chondrocytes, and adipocytes via activating the TGF-β and PKC signaling pathways 40-42. α-MEM contains ascorbic acid compared to DMEM-F12, which stimulates MSC proliferation without affecting phenotype or proliferative potency 43-45, and can also increase the expression of the Sox2 and Oct4 genes 44. Large quantities of cysteine found in α-MEM can shield mitochondria from H2O2-induced oxidative stress, preventing apoptosis, necroptosis, and mitoptosis in human MSCs. Cysteine also improves the adherence of MSC cells both in vitro and in vivo. Previous studies have shown that α-MEM increases the proliferation of mouse and human MSCs more effectively than DMEM and RPMI 46,47. Nekanti et al showed that DMEM-F12 works better than other media for MSC proliferation in vitro 48. Nevertheless, in this study, there was no significant difference in the proliferation rate of cells between these two basic culture media.
Following isolation, to investigate the impact of bFGF, NEA, or L-Gln on the expansion capacity of WJ-MSCs, cells were seeded into 24-well plates at a density of 6×104 cells per well in medium (DMEM-F12 or α-MEM) supplemented with bFGF, NEA, or L-Gln. Although the subculture of MSCs is necessary to obtain sufficient cell numbers, using MSCs at early passage may be beneficial for treatment purposes. Evidence suggests that increasing the passage number of MSCs, reduces their abilities to differentiate 49, and cytokine secretion 50, and improves disease conditions 51. Due to these reasons, it becomes challenging to generate MSCs in sufficient numbers and functionality for therapeutic purposes. To produce WJ-MSCs with high therapeutic potential, it is crucial to develop reproducible culture conditions. Previous studies have indicated that human MSCs exhibit a lack of susceptibility to spontaneous transformations and have demonstrated normal karyotypes and consistent DNA copy numbers during long-term cultures 52,53. Additionally, Nekanti et al showed WJ-MCS retained a normal karyotype even after large-scale expansion, facilitated by bFGF treatment 54. In our study, focus was on evaluating cell viability using Trypan Blue dye. This approach enabled the exclusion of non-viable cells during the cell counting process.
The half-life of bFGF, like that of other FGF family members, is around eight hours under the typical mammalian cell culture conditions (37°C and 5% CO). Because of this instability, using bFGF in cell culture is challenging and frequently necessitates high concentrations of the growth factor, daily media changes, or additional supplementation of bFGF 55.
In the current study, a twenty-four-hour cycle of media changes was followed in all cultures. Differences in the growth rate were apparent as early as 48 hr; however, the highest proliferation rate was observed at 96 hr with bFGF supplementation in both α-MEM and DMEM-F12 compared to the control group. The proliferation rate of human WJ-MSCs cultured in the presence of 10 ng/ml of bFGF was significantly increased compared to those of cultures supplemented with L-Gln and NEA+ L-Gln (Figure 5).
Unlike bFGF+NEA+L-Gln, there was no significant difference between L-Gln and NEA+L-Gln in comparison to the control group, indicating that bFGF was an effective supplement for cell proliferation. So, under the cell culture conditions of the current study, the addition of bFGF to both α-MEM or DMEM-F12 resulted in a significant increase in the proliferation of WJ-MSCs. The mechanism by which bFGF affects cell proliferation may be associated with increased TRPC1 channel activity through ERK activation and altered expression of apoptosis-related proteins 56. Analysis of cell cycle data revealed that bFGF promotes cell proliferation by increasing the proportion of cells spending more time in the S phase and progressing to the M phase upon bFGF stimulation. Additionally, it stimulates the expression of cyclin D proteins and associated kinases. Furthermore, bFGF supplementation influences the cytokine profile of cells and reduces apoptosis 57.
Earlier investigations have primarily revolved around various sources such as bone marrow, adipose tissue, human Amniotic Fluid-derived MSCs (AF-MSCs), or cord blood-derived MSCs 54,58-68. These studies have contributed valuable insights into the enhancement of MSC proliferation. Nonetheless, a notable research gap exists, wherein there remains a lack of dedicated examination pertaining to the influence of specific supplements integrated into fundamental culture media.
This study aims to deliberately promote the proliferation of WJ-MSCs, highlighting an area that warrants further investigation. Furthermore, according to the available literature, MSCs from different sources show a different response to these tested factors since a source-and supplement-dependent variability regarding MSC proliferation and phenotype is already well documented. Besides, previous studies, such as Tesarova et al, investigated the effect of growth factors on the proliferation of WJ-MSC 68. The distinguishing factor between our study and studies of this nature lies in our utilization of the explant method for cell extraction in the initial stage, as opposed to the enzymatic method. The explant method offers several advantages over enzymatic isolation. Notably, it subjects cells to no proteolytic stress, yields high isolation efficiency, and results in reduced costs and contamination risks 36. In a study by Yoon, MSCs from Wharton's jelly were purified using both explant and enzymatic digestion techniques, with the former demonstrating superior viability and cell count 69. The explant method has been reported to yield less heterogeneous cell populations, characterized by heightened proliferation rates and enhanced cell viability, in comparison to the enzymatic method 36,70.
This study demonstrated that the bFGF supports WJ-MSCs cell proliferation more efficiently than L-Gln and NEA. Nevertheless, these findings indicate that although the presence of bFGF significantly improves cell proliferation, proper preparation of Wharton's Jelly of the human umbilical cord tissues can also contribute to the growth and proliferation of MSCs. This culture protocol could improve the efficiency of isolation and preparation of WJ-MSCs.
Conclusion :
The explant method presents an accessible and cost-effective approach for the isolation, cultivation, and amplification of WJ-MSCs, all while preserving their stemness characteristics, including self-renewal capacity, multi-differentiation potential, and distinct surface markers. The swift cellular expansion and robust proliferation exhibited by WJ-MSCs hold the significance of generating larger cell numbers, a crucial aspect for repetitive administrations. This study delves into the impacts of bFGF, non-essential amino acids, and L-Glutamine within α-MEM and DMEM-F12 culture media on the expansion prowess of WJ-MSCs.
Furthermore, these findings underscore the viability of utilizing bFGF as a supplemental component in culture media, effectively refining and augmenting the proliferative competence of human WJ-MSCs. In light of these results, it is advisable to undertake further investigations to scrutinize differentiation and regeneration potential, as well as to dissect the activation of diverse signaling pathways in MSCs, based on the varied treatments employed in this experimental setup.
Ethical Considerations :
All Experimental procedures were conducted according to the guidelines of the Ethics Committee of Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Ethical code: IR.SBMU.RIGLD.REC. 1399.062).
Acknowledgement :
Not applicable.
Funding: This research was supported by the Gastroenterology and Liver Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Conflict of Interest :
The authors declare that there is no conflict of interest.
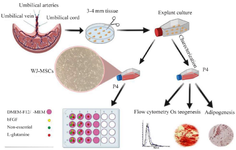
Figure 1. Schematic illustration of the workflow. Characterization of mesenchymal stem cells isolated from human umbilical cord-derived Wharton’s jelly by explant method. On the lower left, DMEM-F12/α-MEM only means one of them. Therefore, the experiment was performed once with DMEM-F12 with supplements and once in α-MEM culture medium treated with supplements.
|
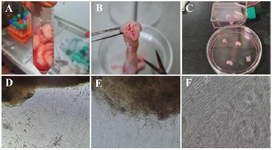
Figure 2. Isolation of WJ-MSCs by explant method. A) The whole umbilical cord, B) The cross-section of the umbilical cord and the umbilical cord blood vessels, C) Wharton's jelly cut into 3–4 mm explants. Representative phase-contrast images of the WJ-MSCs; D) The morphology of primary cells that migrated from explant tissue day 7, E) The morphology of cells after 10 days, F) and after 14 days, WJ-MSCs were grown from the explants.
|
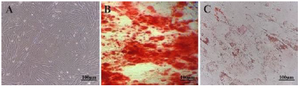
Figure 3. Characterization of Wharton's jelly-derived mesenchymal stem cells (WJ-MSCs) (A) Spindle-shaped, fibroblastic-like mesenchymal stem cells derived from human umbilical cord WJ-MSCs (B) Osteogenesis differentiation assay with alizarin stain revealed the formation of calcium oxalates in differentiated MSCs. (C) Oil-red O intracellular staining for representation of adipogenic differentiation of WJ-MSCs.
|

Figure 4. Flow cytometry Analysis of WJ-MSCs cell surface markers. Negative markers and positive markers for CD73, CD90, and CD105.
|
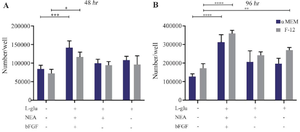
Figure 5. Proliferation capacity of isolated WJ-MSCs in different cell culture media and time intervals. A) WJ-MSCs, which were cultured in α-MEM basal medium with bFGF+NEA+L-Gln altogether, showed a better proliferation effect compared to the control (p=0.0002) during 48 hr culture (p=0.0002). At this point, in DMEM-F12 culture media supplemented with the same treatments, only bFGF+NEA+L-Gln treats were significantly higher than the control. B) After 96 hr of incubation with mentioned supplements, three treats presented a significantly better growth rate than the control, irrespective of their basic media. After 96 hr, the same proliferation pattern in 48 hr was detected but at a higher rate.
**** p<0.0001, ***p<0.001, ***p<0.001, **p<0.01 and * p<0.05. Non-significant is not shown in the figure.
|
|