Differentiation of Human Umbilical Cord Matrix-Derived Mesenchymal Stem Cells into Germ-Like Cells
-
Latifpour, Mostafa
-
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Shakiba, Yadollah
-
Department of Immunology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Amidi, Fardin
-
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Mazaheri, Zohreh
-
Department of Anatomy, School of Medicine, Tarbiat Modares University, Tehran, Iran
-
 Sobhani, Aligholi
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 21 66597378; Email: sobhania@tums.ac.ir
Sobhani, Aligholi
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 21 66597378; Email: sobhania@tums.ac.ir
-
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Abstract: Background: Mesenchymal Stem Cells (MSCs) are multipotent cells that can be collected from different sources. Under specific conditions, MSCs can be differentiated to tissue specific cells in vitro. Human Umbilical Cord Mesenchymal Stem Cells (hUCMSCs) can easily be harvested and cultured in in vitro conditions. Production of germ cells from mesenchymal stem cells is a very interesting and promising area in the field of reproductive medicine. In the present study, the possible trans-differentiation of hUCMSCs into Primordial like Germ Cell (PGC) was performed in vitro under specific condition. Methods: Human umbilical cord mesenchymal stem cells were cultured and expanded in DMEM medium containing 10% FBS. The cultured cells were studied for differentiation ability to adipocytes and osteocytes. Furthermore, MSCs related markers were identified by flow cytometry method. For PGC differentiation, hUCMS cells were cultured in differentiation medium containing Bone Morphogenetic Protein 4 (BMP4) and it was followed by retinoic acid (RA). Real time PCR and immunocytochemistry analysis were performed to evaluate the expression of PGC specific genes and proteins, respectively. Results: Our results showed that hUCMSCs cultured in the presence of BMP4 and RA are able to transdifferentiate in to PGC like cells in vitro. Real time PCR and immunocytochemistry results showed that differentiated cells expressed PGC specific markers after 14 days of culture. Conclusion: Based on these results, it was concluded that hUCMSC may be considered as a promising alternative cell source in reproductive medicine. More studies including laboratory and also animal models are needed to evaluate the functionality of differentiated PGCs before introducing them to clinical applications.
Introduction :
World Health Organization (WHO) defines infertility as the inability of the couples in reproductive age to achieve pregnancy after 12 months of unprotected intercourse. Based on the 2005 National Survey on Family Growth American report, this condition affects 13-15% of the couples worldwide 1. This difference may be attributed to disparity in the resources allocated for diagnosis and treatment of infertility. New methods for overcoming therapeutic problems in this filed are highly desired.
Mesenchymal Stem Cells (MSCs) are unique in their multipotent differentiation ability. Based on collected sources, MSCs can be categorized as embryonic and adult stem cells. Adult stem cells can be collected from a variety of sources including bone marrow, adipose tissue, skin tissue and synovial membrane 2. In addition to embryos and adult tissues, MSCs can be collected from extra-embryonic tissues including placenta, amniotic membrane and fluid 3 and Umbilical Cord Matrix (UCM) 4. These cells can be harvested easily by non-invasive procedures, do not have ethical issues and do not induce immune response in the host animals 5. Recent studies demonstrated that human Umbilical Cord Mesenchymal Stem Cells (hUCMSCs) are multipotent cells with immunomodulatory properties 6-8. In recent years, many animal studies showed that hUCMSCs can have a therapeutic use in different tissue injuries 9-11. Interestingly, it has been shown that hUCMSCs can restore ovarian function and reduce apoptosis in mice model of premature ovarian failure 12.
Previous studies showed that Embryonic Stem Cells (ESCs) from mouse and human sources can develop into the germ cell lineage 13,14. In addition, several reports have recently documented Primordial Germ Cell (PGC) and sperm-like cell development after ESC differentiation, and one study observed mature and functional mouse ESC-derived sperm that were capable of producing offspring 15,16. In the present study, the possible transdifferentiation of hUCMSCs into PGC like cells was studied in vitro.
Materials and Methods :
This study was performed in accordance with the guidelines for the care and use of laboratory human established by the local ethics committee at Tehran University of Medical Sciences, Tehran, Iran. All chemicals were purchased from Sigma-aldrich Chemical Company (St. Louis, MO, USA).
Isolation and culture of human umbilical cord matrix cells: The umbilical cords were obtained from newborns delivered by cesarean section by healthy mothers, without any clinical problems. Immediately after delivery, sections of the umbilical cords were gathered and transported to the laboratory in room temperature in sterile Hank’s balanced salt solution contained 40 µg/ml of gentamicin. In the laboratory, the cords were chopped into 3 to 5 cm lengths, then pieces were rinsed several times with sterile phosphate buffered saline containing 2 μg/ml amphotericin B, 100 U/ml penicillin, and 100 μg/ml streptomycin. To isolate hUCMSC cells, the amnion and cord vessels were carefully removed and the Wharton's jelly was diced into 2-5 mm explants. The fragments were then transferred into 25 cm2 tissue culture flask containing 2 ml of DMEM supplemented with 15% fetal bovine serum, 100 U/ml penicillin and 60 μg/ml streptomycin, placed in an incubator in humidified atmosphere at 37°C and 5% CO2 in air.
After 7 days when hUCMSCs were grown from the periphery of the fragments, tissue explants were removed. The hUCMSCs were observed daily with the microscope to assess the cells proliferation. hUCMSCs at confluence of > 90% were either sub-cultured or cryopreserved with 10% Dimethylsulfoxid (DMSO) and 90% FBS for further use. For cryopreservation, cell suspensions (1×106 cells/ml) were prepared in 1.8-ml cryovials (Greiner bio-one, Germany) containing 1 ml freezing medium, placed in an insulated (Nalgene Mr. Frosty freezing) container at -80 ºC for one day and then plunged into liquid nitrogen.
hUCMSCs were used at passages 2-5 for experiments. The 90% confluency was used for cell passage but for colony formation, it was necessary to continue the culture time.
Phenotype analysis of hUCMSCs: To evaluate alkaline phosphates activity in hUCMSCs, 1×106 cells at passages 2-3 were cultured in 24-well cell chamber slide (TC Testplate) (Greiner bio-one, Germany) until colonies appeared and then the colonies were stained with an alkaline phosphates kit (R-87; Sigmaaldrich, St. Louis, USA) (Figure 1).
Flow cytometric analysis: Cell surface markers analysis was performed using flow cytometry method. One million cells/ml at passages 2-3 were labeled with the following antibodies for 20 min: PE-conjugated anti-CD45, PE-conjugated anti-CD34, FITC-conjugated anti-DR, FITC-conjugated-anti-CD73, FITC-conjugated anti-CD90, PE-conjugated anti-CD105 (all eBioscience, USA). The cells were analyzed using FACSCalibur (Becton Dickinson) machine and WinMDI Cellquest software.
Induction of osteogenic and adipogenic differentiation: To evaluate the differentiation potential of hUCMSC cells, confluent cells at passages 2-4 were cultured in differentiation mediums for induction of osteocyte and adipocyte like cells. Adipogenic differentiation medium was consisted of DMEM containing 10% FBS and 100 nM dexamethasone. Osteogenic differentiation medium consisted of DMEM containing, 10 nM β-glycerophosphate, 80 µg/ml ascorbic acid and 10 nM dexamethasone. Human umbilical cord mesenchymal stem cells cultivated in basal medium with 10% FBS served as the control. The medium was changed every 4 days. Twenty-one days later, the cultures were used for histochemical staining such as Oil Red and Alizarin Red S. (all Sigma, St. Louis, USA).
Induction of PGC differentiation: For PGC differentiation, confluent hUCMSC cells at passages 3-6 were cultured at a concentration of 1×106 cells in 25 cm2 culture flasks (Falcon, USA) at 37C in humidified atmosphere with 5% CO2 in differentiation medium containing 20 ng/ml bone morphogenetic protein 4 (BMP4; Sigmaaldrich, St. Louis, USA) for 4 days. After that, Retinoic Acid (RA) (10-5 M) was added for a culture period of 14 days. The medium was refreshed every 2-4 days. Human umbilical cord mesenchymal stem cells were allocated into 2 groups: (1) intact group and (2) control group.
RNA isolation and Real time PCR: Total RNA was isolated from treated cells with BMP4 and hUCMSC cells as controls using TriPure isolation reagent. In order to remove genomic contamination, RNA was treated with DNase I using a kit from Fermentase (Lithuania) based on the protocol described by the manufactures. Concentration of RNA was determined using UV spectrophotometer (DPI-l, Qiagen). The quantity of extracted RNA was assessed using a Nanodrop instrument. The quality of RNA was evaluated by calculating the 260/280 nanometer ratio. The cDNAs were synthesized from 1000 ng DNase treated RNA sample with a Revert AidTM first-strand cDNA synthesis kit (Fermentase, Lithuania) using oligo (dT) primers. All primers/probes (DDX4, STELLA, Oct4, SCP3 and 18S rRNA as internal control) and also master mix were from Applied Biosystems (Applied Biosystems, Foster City, CA, USA). The PCR was performed according to the manufactures recommendations. Briefly, each PCR reaction contained 1000 ng cDNA, 12.5 µl TaqMan gene expression master mix, 0.6 µl of predesigned primers and probes in a total volume of 25 µl. The PCR cycles were 50C for 2 min, 95C for 10 min, followed by 45 cycles of 95C for 15 s and 60C for 1 min. PCR was performed using 96 well plates on ABI StepOnePlus instrument (Applied Biosystems, Foster City, CA, USA). The results were determined using StepOne software V2.1 (Applied Biosystems, Foster City, CA, USA). Expression levels of genes, according to 2-ΔΔCt formula were calculated. The data were analyzed by SPSS using ANOVA test and the data was indicated as the mean±SD. The level of significance was assumed as p≤0.05.
Immunocytochemistry of in vitro differentiated cells: For immunofluorescent localization of germ-cell markers, treated cells with BMP4 and hUCMSC cells as controls were cultured on pre-washed sterile coverslips under the previous conditions. The cells were washed 3 times with PBS and fixed for 30 min in 4% paraformaldehyde at room temperature. The fixed cells were incubated for 25 min with a 1:9 dilution of normal goat serum in PBS to block nonspecific binding of the primary antibody. The cells were incubated in 3% H2O2 in PBS for 15 mins to block endogenous peroxidase and then incubated with anti Oct4, Nanog, SSEA4, DDX4 and C-kit antibody overnight at 4C. The slides were washed 3 times with PBS and incubated with goat anti-rabbit IgG-FITC (all from Abcam, UK) for 1 h at room temperature. Cells were washed and incubated with DAPI (Sigmaaldrich, St. Louis, USA) for 5 mins to detect the nuclei. Cells were evaluated under an Olympus phase contrast microscope (BX51, Olympus, Tokyo, Japan).
Statistical analysis: The one-way ANOVA and Tukey post-hoc tests were used to determine the statistical significance of observed differences in the mean values among our groups using the SPSS (SPSS 16.0 production mode facility). The data are presented as mean±SD. Each data point represents the average of three separate experiments with three repeats in each experiment. The p-value less than 0.05 indicated statistical significance.
Results :
hUCMSCs culture and cell marker expression: Human umbilical cord mesenchymal stem cells were isolated from 5 cords by the culture method, as described previously. The isolated cells demonstrated a fibroblast-like phenotype (Figure 2). Human umbilical cord mesenchymal stem cells were maintained in culture until colony formation was observed. Human umbilical cord mesenchymal stem cells expressed alkaline phosphatase after colony formation both in culture plate (Figure 1). Flow cytometric analysis showed that the cells expressed mesenchymal stem cell markers (CD73, CD105 and CD90), and did not express hematopoietic cells surface markers (CD34 and CD45), and DR marker (Figure 3). Adipogenic differentiation, as seen by Oil Red O-positive cells (Figure 4), and osteogenic differentiation, as seen by Alizarin Red S-positive cells, was achieved by culturing the cells for twenty one days in differentiation mediums, as described previously (Figure 5).
PGC differentiation of hUCMSC cells: Real time PCR analysis: Expression of germ cell specific genes such as OCT4, STELLA, SCP3 and DDX4 was detected in treated and control hUCMSC groups by Real time PCR analysis. Treated cells after an extended culture period were transformed into slender spindles and were strongly positive for Stella, as a germ cell marker during early development in treated group in the initial induction, but expression of this gene in the control group was not observed. Expression of Oct4, considered as a marker of pluripotency, was detected in all cell populations. The meiosis-specific SCP3 transcript was detected in treated cells after 14 days just like the germ cell specific transcript for DDX4 protein and Stella as a differentiation and maturation agent of PGCs, which is known to be expressed in post-migratory primordial germ cells until the postmeiotic stage of PGC like cells. No expression could be seen in the primary treated cells after 7 days.
Immunocytochemical evaluations: Different markers typical of stem cells were tested and specific stages of germ cells by immunocytochemical staining were evaluated. Immunocytochemical staining for the transcription factors such as Oct-4 and Nanog were positive in the control cells and in primary treated cells after 7 days (Figures 6 and 7), whereas only faint staining could be detected in the treated cells after 14 days (Figure 8). Analysis of SSEA4 (potent germ cell marker) expression showed distinct differences in the analyzed cell populations. In primary treated cells after 7 days, staining for this surface marker could hardly be demonstrated, but in the treated cells after 14 days, prominent staining of the cells was found (Figure 9). To analyze further germ cell characteristics of treated cells, the expression of two additional germ cell markers was examined. The transcription factors such as DDX4 were expressed in post-migratory primordial germ cells until the premeiotic stage of germ cells. Expression of DDX4 was present in the treated cells after 14 days (Figure 10), whereas it couldn't be detected in control and primary treated cells after 7 days.
Discussion :
The isolation of stem cells from human umbilical cord matrix was previously reported. These stem cells possess properties of MSCs and can differentiate adipogenic, osteogenic and cardiogenic like cells, and engrafted hUCMSC cells could experimentally improve infarcted myocardium function after 30 days 10. This study demonstrates that BMP4 treated cells could differentiate into PGC-like cells after 14 days in vitro. It was documented that stem cells from e.g. human and murine embryo 13,14, bone marrow 15 or pancreas 17 are able to differentiate into all three germ layers. Nevertheless, the generation of germ cells seemed to be exclusively possible for embryonic stem cells 13,14 and there was only weak evidence of transdifferentiation of adult stem cells into PGC like cells 15,17. Human umbilical cord mesenchymal stem cells are able to significantly express germ cell specific genes. Overall, two different methods have been used for germ cell differentiation from human and murine ESCs. One possible method is spontaneous differentiation in adherent culture after removing factors that promote pluripotency as feeders and Leukemia Inhibitory Factor (LIF) or basic Fibroblast Growth Factor (bFGF) 16,18 whereas the second one implicates the formation of three-dimensional structures known as Embryoid Bodies (EBs) 13,19,20. In the present study, the second method was used for differentiation of hUCMSC cells.
An ideal source of donor cells for therapy of reproductive diseases should be identified and the cells should be collected and rapidly expanded in the laboratory. Moreover, they should be immunologically compatible and capable of long-term survival and integration in the host tissue. Human umbilical cord mesenchymal stem cells from human Wharton's jelly of umbilical cord can be easily obtained and harvested compared to Bone Marrow Stem Cells (BMSC). These cells have greater Colony Forming Unit-fibroblast (CFU-F) frequency and shorter doubling time as compared to BMSCs 21,22. An important difference between hUCMSC and BMSCs is that hUCMSC cells can be easily isolated from most of the samples, even from umbilical cords that are delayed in their processing up to 48 hr 23.
In the present study, a non-enzymatic method was used which yielded sufficient number of cells after 7-10 days. These cells propagated rapidly in a common culture medium (DMEM F12 and 10% FBS), without any need to specific culture supplements. Isolated cells formed alkaline phospahatase positive colonies in plastic dishes and hanging drops, a feature common to embryonic stem cells 14. These cells were positive for embryonic stem cell marker Oct4 but uniformly negative for CD117. These hUCMSC cells expressed matrix receptors CD44, CD90 and CD105 and uniformly did not express hematopoietic lineage markers CD34 and leukocyte common antigen CD45. These features suggest that human umbilical cord matrix-derived mesenchymal cells are more similar to mesenchymal stem cells 24. Hence, the results show that large amount of mesenchymal stem cells could not easily be harvested from human umbilical cord matrix for the treatment of reproductive diseases, which might become an ideal source of stem cells for cell therapy in reproductive disease through Assisted Reproductive Technology (ART). In agreement with other previous studies 15,17, in this study, hUCMSCs were successfully differentiated to PGC-like cells after 4 days of incubation with BMP4 and RA and it was followed by two weeks of culture in the laboratory. The addition of exogenous factors such as BMP-4, BMP-7 and BMP-8 to stem cell cultures increases the number of germ cells expressing DDX4, but does not necessarily induce their progress in meiosis 25. After PGC like cells differentiation, morphological changes were observed every day under a phase contrast microscope. After 7 days of incubation, the wide or polygonal cells almost disappeared and the cells appeared as slender spindle shaped that this morphology did not change significantly up to 14 days of treatment. The treated cells have a looser density than untreated cells in basic culture medium. The control group of untreated hUCMSC was 100% confluent on day 14. A close morphological observation of treated cells revealed similarities to in vitro generated PGC like cells from embryonic 18,19 and adult stem cells 26.
To prove putative differentiation of hUCMSC cells into germ cells, the expression analysis of stem cell markers as well as germ cell markers was performed as compared to primary hUCMSC cell populations. Real time PCR analysis of the stem cell markers DDX4 and SSEA-4, which are potent germ cell markers as well, could be clearly detected in PGC like cells, whereas the primary hUCMSC cell populations showed only faint staining for these markers. Regarding Oct-4 expression, increased protein was found by immunocytochemical staining in PGC like cells. Similarly, expression of SSEA-4 was present in hUCMSC cells and could be strongly detected in the PGC like cells. Also, DDX4 is specific for differentiating germ cells from the late migration stage to the postmeiotic stage 27 and it was expressed in PGC like cells and detected by Real time PCR and immunocytochemical staining.
A recent study by Nayernia et al showed that a cocktail of soluble growth factors, including RA are able to sustain the survival and self-renewal of mouse germ cells in the absence of somatic cell support 16. After germ cell adhesion to an acellular substrate, such growth factors and compounds were able to prevent the occurrence of significant levels of apoptosis in germ cells, stimulate their proliferation and allow most of them to enter into and progress through meiotic prophase I 28,29. Furthermore, RA is known to promote the developmental progression of germ cells 30.
Therefore, in this study, RA was used in our efforts to direct functional gametogenesis in vitro. For female germ cell differentiation, both cultures in monolayer and through EBs formation succeeded although the oocyte-like cells seemed to be in an immature stage, in most cases with a fragile structure. Their use for the generation of live progeny has not been reported. The addition of exogenous factors to the culture media such as BMPs or RA which play basic roles in germ cells and gametes development in vivo, seem to help to expand the germ cell population and push them to the meiotic process in vitro, but it is not enough to direct them through that process properly 31. At this point, a gonadal-like three-dimensional structure or specific cell-to-cell contact might be required to progress through meiosis and to acquire a correct epigenetic status. An environment similar to the in vivo niche might be a necessary requisite.
Mesenchymal stromal cells have a relatively immuno-privileged phenotype, enabling them to survive and reside in inflammatory milieu without activating immune system. This is somewhat because of lack of surface expression of histocompatibility complex class II antigens in the resting state and also their lack of co-stimulatory molecules for T cell induction (CD40, CD40 ligand and the B7 molecules CD80 and CD86) 32. Similar to MSCs, hUCM cells express HLA-class I but not HLA-class-II surface markers 33. However, other researchers have implanted umbilical cord matrix mesenchymal cells as xenografts and reported that this procedure did not alleviate immune response in the host animals 34.
In agreement with other studies which have utilized umbilical cord-derived matrix stem cells for the treatment of degenerative diseases, our findings suggest that hUCMSC cells can develop into PGC like cells with change in morphology and typical expression of various germ cell markers.
hUCMSC as an easily acquired, inexpensive source of cells, propagate rapidly in vitro and have potential of differentiating to germ like cells in vitro. These properties and their nonimmunogenic nature of these cells suggest them as an alternative, valuable candidate for cell therapy in degenerative reproductive diseases. However, some debates remain to be elucidated before these cells could be utilized in clinical studies. The safety of hUCMSC as a heterograft tissue, long term survival of these cells, optimal cell dose, route of delivery, potential of hUCMSC cells in treatment of different types of infertility failure and the ability of hUCMSCs in comparison to other cell types are unclear yet. As already shown for hUCMSCs, differentiation into various cell types such as osteocytes, adipocytes, chondrocytes, hepatocytes and neuronal or glial cells is possible. On the basis of this developmental plasticity, it is not implausible that hUCMSCs also give rise to germ cells.
Conclusion :
The hUCMSC may be considered as an alternative cell source to repair reproductive disease. These cells could differentiate into PGC like cells under the influence of BMP4 and RA. Therefore, clinical application of hUCMSC has been considered promising for regenerative medicine.
Acknowledgement :
This project financially supported by research deputy of Tehran University of Medical Sciences and Health service, grant No. 91-01-30-17023.
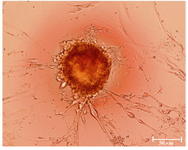
Figure 1. The cultured hUCMS cells formed numerous colonies that stained positively by alkaline phosphatase
|
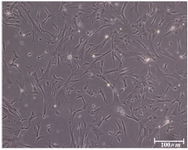
Figure 2. The hUCMS cells retained a fibroblastic cell shape with numerous cytoplasmic processes
|

Figure 3. Flow cytometric analysis of surface markers of hUCMSC cells
|
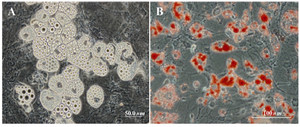
Figure 4. A) Fatty acid droplets after culture in adipogenic medium (phase-contrast image); B) Fatty acid droplets after culture in adipogenic medium (oil red staining)
|
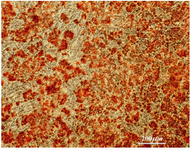
Figure 5. Osteocyte differentiation of hUCMSCs in specific media
|
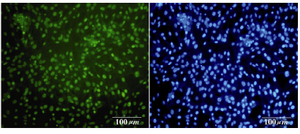
Figure 6. Immunofluorescence location of germ-cell-specific marker Oct4 in differentiated cells after 14 days treatment. Nuclei are shown in blue, DAPI staining
|
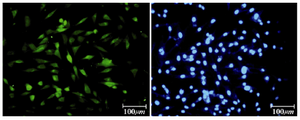
Figure 7. Immunofluorescence location of germ-cell specific marker, Nanog, in differentiated cells after 14 days treatment. Nuclei are shown in blue, DAPI staining
|
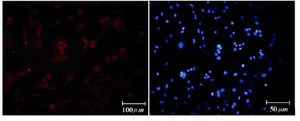
Figure 8. Immunofluorescence location of germ-cell specific markers, Ckit, in differentiated cells after 14 days treatment. Nuclei are shown in blue, DAPI staining
|
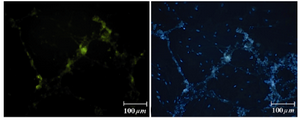
Figure 9. Immunofluorescence location of germ-cell specific marker, SSEA4, in differentiated cells after 14 days treatment. Nuclei are shown in blue, DAPI staining
|
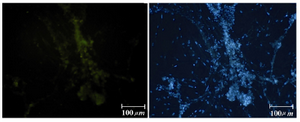
Figure 10. Immunofluorescence location of germ-cell-specific marker, DDX4, in differentiated cells after 14 days treatment. Nuclei are shown in blue, DAPI staining
|
|