Effect of Peptone Feeding on Transient Gene Expression Process in CHO DG44
-
 Davami, Fatemeh
Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran, Tel: +98 21 66953315; Email: f_davami@pasteur.ac.ir
Davami, Fatemeh
Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran, Tel: +98 21 66953315; Email: f_davami@pasteur.ac.ir
-
Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
-
Eghbalpour, Farnaz
-
Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
-
Islamic Azad University of Arak, Arak, Iran
Abstract: Background: Transient Gene Expression (TGE) gained popularity over the last decade as a rapid method for the production of milligram to gram quantities of recombinant proteins for preclinical studies in biophama industry. Thereby, the optimization of the TGE technique for Chinese hamster ovary (CHO) as the dominant host for the production of biotherapeutics is of great interest to reach the values for Human Embryo Kidney-293 (HEK-293) cells in terms of transfection efficiencies and production titers. TGE efficiencies are cell line and vector dependant.
Methods: In transfection efficiency optimization experiments, different starting cell densities, different amounts of plasmid DNA and PEI transfection reagent were investigated to achieve the best conditions leading to maximum transfection efficiencies. Furthermore, in order to investigate the effect of peptone feeding on transfection efficiency, three different sources of peptones with the greatest effect in the CD DG44 basal media were selected; Casein Tryptone N1, Soy petone A2SC and Soy peptone E110.
Results: The transfection strategy performed here was able to make an outstanding increase in transfection efficiency of CHO DG44 cell line transfected with pTracer-SV40-mutated t-PA plasmid from 3.6% in our starting non-optimized condition to 66.93% in finally optimized situation. Moreover, peptone feeding strategy used here was successful to increase volumetric productivities up to 37%. In addition, the amounts of both PEI and plasmid DNA were reduced up to 66% and 25% respectively compared to our previous protocol.
Conclusion: Here we described an optimization process for TGE in suspension-adapted CHO cells based on Polyethylenimine (PEI)/DNA concentration, DNA: PEI ratio, starting cell densities and peptone feeding strategy.
Introduction :
Transient Gene Expression (TGE) is a well-established technology for rapid production of milligram to gram quantities of recombinant proteins in Human Embryonic Kidney (HEK) and Chinese Hamster Ovary (CHO) cell lines 1-3. Due to quick turnover and low cost, TGE platform plays more important role in biopharmaceutical early development stages for two reasons; Firstly, it is essential for large biopharmaceutical companies to screen multiple drug candidates prior to moving forward into the formal development pipeline. Secondly, all the strategies used to optimize expression in a stable cell line development can be utilized and optimized in TGE to evaluate their feasibility before allocating significant resources to generate a stable cell line.
However, in terms of production level, TGE tends to be lower than stable gene expression. While in TGE the relatively low expression level of 1-100 mg/l is usually achieved, this amount goes to 5 g/l with stable cell lines after amplification and selection steps. Thereby, it is still of great importance to further optimize TGE conditions in order to
have higher yields of production.
Comparing two commonly used cell lines for TGE technology, in few studies the expression level of transiently transfected HEK cell line has been reported to reach 1 g/l titers 4. Nevertheless, the highest reported volumetric yields for CHO cells have been 80-100 mg/l 1,5,6.
Considering the fact that CHO cells are the dominant hosts for recombinant protein production in today’s biopharma industry, it would be helpful to optimize the TGE approach for this cell line in order to have the potential to extrapolate the data for stable cell line development.
Due to the many disadvantages associated with the use of serum in cell cultures, industrial bioprocesses are now essentially based on Serum-Free Media (SFM) and more generally animal-components free media, which have both economic and safety advantages over animal-derived products 7-10.
The need to minimize animal-derived components in the production process has created an interest in using protein hydrolysates (peptones) as alternative supplements to replace serum. Peptones are water-soluble, protein hydrolysates of non chemically-defined nature, containing peptides, amino acids, and inorganic salts, but devoid of lipids and sugars 11. This category of media components are low-cost, growth-promoting nutrients for intensive animal cell culture that supply nutrients or growth factor analogues based on degree of hydrolysis 2,12. Various peptones are commercially available such as digest of beef tissues, meat, casein, lactalbumin, and yeast. The main disadvantage of most of these peptones is their animal origin which endangers medium biosafety 7.
Supplementation of standard protein-free media with peptones has shown to cause a significant increase in TGE productivity in HEK 293-EBNA cells 13,14. This effect has also been studied in CHO stable cell lines 15,16. However, to our knowledge, no study has yet been done to investigate the effect of peptone supplements on CHO cells in transient expression of recombinant protein.
In this study, efforts were made to identify the best TGE condition regarding PEI, DNA concentration and cell density. Moreover, a feeding strategy was applied for the recombinant CHO DG44 cell line to produce a novel chimeric-truncated form of tissue plasminogen activator.
Materials and Methods :
The chemically defined serum-free medium used was denominated CD DG44 from Invitrogen (GIBCO Invitrogen, USA). Peptones were supplied from Organotechnie (La Courneuve, France) and the company provided the total amino acid composition, molecular weight distribution, and free amino acid content of the peptones (Table 1). Linear PEI 25 kDa was purchased from Polysiences, (Eppelheim, Germany). Chromolize t-PA Assay Kit was purchased from Biopool (Trinity Biotech plc., Ireland).
Cell cultivation: Suspension-adapted CHO-DG44 cells were routinely cultivated in CD DG44 medium supplemented with 13.6 mg/l hypoxanthine, 3.9 mg/l thymidine, and also 6 mM glutamine. Cultures were agitated at 110 rpm in TubeSpin® Bioreactors on an orbital shaker (at 37ºC in 5% CO2 atmosphere) 17. The cultures were inoculated with cells from the mid-exponential phase of growth at a cell concentration of 0.20×106 cells/ml.
Cell number: Cells density and viability were assessed by the trypan blue dye exclusion method using a haemocytometer (Neubauer improved, Brand). Cell viability was determined by the trypan blue exclusion method (1:1 mixture of 0.2% trypan blue in normal saline and cell sample). After cell counting, the remainder of each sample was centrifuged (5000 g, 1 min) to remove the cells and the supernatant was frozen for further protein production analysis. The error bars in graphs of cell viability represent the standard deviation from three independent experiments and are only intended to show the similarity between the experiments.
Plasmid: PTracer-SV40-truncated mutant t-PA, the product of cloning mutant t-PA gene in pTracer-SV40 was studied in our previous research 18-20. The recombinant pTracer-SV40-mutated t-PA plasmid was expanded in Escherichia coli Top 10 and purified using Qiagen EndoFreeTM Giga-prep kits (Quiagen GmbH, Hilden, Germany). PTracer-SV40 is a CHO expression vector that uses SV40 promotor and encodes Green Fluorescence Protein (GFP). Plasmid was stored in endotoxin-free water. Purity and concentration of the plasmid DNA were determined by measuring the absorption at 260 nm and 280 nm in a spectrophotometer. The A260/A280 ratio was typically between 1.80 and 1.90.
Transfection: Linear PEI 25 kDa (Polysiences Eppelheim, Germany) was used as the transfection reagent for all transfections. All transfection experiments were done in triplicates at a final volume of 5 ml in TubeSpin® Bioreactor 50 ml tubes (TubeSpin; TPP, Trasadingen, Switzerland).
High cell density transfection protocol was performed as a starting point based on previous studies showing improved transfection efficacy with this technique in HEK293 4, and DG 44 cells (TGE protocol yashas).
The control transfection was performed as follows. One day prior to transfection, cells were cultivated in fresh CD DG44 medium at a cell density of 1×106 cells/ml. On the day of transfection, the cells were centrifuged and resuspended in 2.5 ml of pre warmed CD DG44 medium at 2×106 cells/ml in 50 ml of TubeSpins®. For final volume of 5 ml culture, 6.25 μg of DNA (PTracer-SV40-truncated mutant t-PA) and 25 μg of PEI were separately diluted in 0.125 ml of NaCl 150 mM. These two DNA and PEI transfection reagents were then mixed together, and incubated for 10 min at room temperature to allow DNA-PEI polyplex formation. The polyplex was then added to the cells at a defined concentration, and culture incubation was performed for 4 hr in a CO2 shaker incubator at 37C with 5% CO2 and 85% humidity agitated at 180 rpm. The transfections were done at 37C, following a temperature shift to 31C, 4 hr posttrans-fection at the same time with diluting the cells with fresh medium to a final cell density of 4×106 cells/ml. The transfection efficiency (% of GFP-positive cells) was determined by flow cytometry 48 hr after transfection to allow enough accumulation of the GFP protein for optimal detection.
In transfection efficiency optimization experiments, different starting cell densities (the cell density at the time of transfection) and different amounts of plasmid DNA and PEI transfection reagent were investigated to achieve the best conditions for maximum transfection efficiencies.
Peptones: In order to investigate the effect of peptone feeding on transfection efficiency, based on our previous studies (unpublished data), three different sources of peptones with the greatest effect in the CD DG44 basal media were selected; Casein Tryptone N1, Soy petone A2SC and Soy peptone E110. Peptone stock solutions were prepared (20%, w/v), sterilized by filtration through 0.22 µm filters, and stored at 4C. On the day of transfection, each individual peptone was added to the final concentration of 1 g/l for each transfection tube. The results of peptone feeding were compared to a non-fed (control negative) parallel test.
Flow cytometry: Flow cytometry was used for the analysis of transfection efficiency. GFP-specific fluorescence was measured 48 hr posttransfection using PAS flow cytometry (Partec, Germany). GFP fluorescence was excited at 485 nm and emission measured at 530 nm. Viable cells were gated by forward and side scatter using Cell Quest Pro software in order to be separated from cell debris. The gated cells were then analyzed for the quantity of GFP expression. GFP positives were determined by comparing them with a negative control of parallel untransfected culture. The error bars in all figures concerning transfection efficiency (% GFP-positive cells) represent the standard deviation from three independent experiments.
Protein analysis: The truncated-mutant t-PA concentration in the culture medium was determined by an ELISA based technique. The amidolytic activity test (Biopool) entitled Chromolize t-PA Assay Kit is a biofunctional immunosorbent assay based on capturing t-PA by sp-322 monoclonal antibodies coated on the microtest wells. After fulfilling, the steps from the kit’s manual samples were read at 405 nm and 492 nm. Absorbance at 492 nm is measured and subtracted from 405 nm. Various dilutions of each sample were assayed. The amount of developed color was proportional to the amount of t-PA activity in the sample.
Results :
The transfection efficacy in the control protocol (mentioned in Materials and Methods section) was 3.6% with 0.627 IU/ml t-PA expression level (unpublished data).
Optimization of the PEI amount: The cells were transfected at a density of 2×106 cells/ml with different amounts of PEI and constant amounts of 1.25 μg/106 cells of plasmid DNA. The maximum transfection efficiency was achieved with 1.5 μg/106 cells of PEI followed by 1 μg PEI/106 cells with 28.3 and 12.6% transfection efficiency respectively (Figure 1A). Interestingly, further increase in the PEI amounts decreased the transfection efficiency (Figure 1A).
The recombinant protein production level was also higher with 1.5 μg/106 cells (0.75 IU/ml), followed by 0.6297 IU/ml for 1 μg/106 cells of PEI concentration (Figure 1B). There-by, for the following experiments, the amount of PEI concentration was set to 1.5 μg/106 cells.
Optimization of the starting cell density: In order to find the optimum starting cell density on the day of transfection, cells transfected at different cell densities were tested keeping the amounts of DNA and PEI constant. For each transfection, cells were centrifuged and resuspended in various amounts of CD DG44 medium to achieve the cell densities indicated. The cells were transfected by addition of 1.25 μg DNA/106 cells and 1.5 μg PEI/106 cells followed by incubation at 31C. At 3-4 hr posttransfection, the cultures were diluted to 1×106 cells/ml and incubated at 31C. Regarding the transfection efficiency, cell densities of 0.2×106, 0.5×106 and 1×106 cells/ml resulted in significantly higher amounts of 34.715, 37.32 and 34.82% transfection efficiency as compared to higher starting cell densities (Figure 2A). However, among the various transfection conditions, there were no significant differences in volumetric productivity of day 9 posttransfection (Figure 2B).
Optimization of the plasmid DNA amount: To determine the amount of plasmid DNA needed for transfection under the conditions optimized above, cells at a density of 0.5×106 cells/ml were transfected with various amounts of DNA while the PEI amount was kept constant at 1.5 μg/106 cells.
Although no significant differences in transfection efficiency were observed between various amounts of DNA, the highest transfection efficiency (with 0.5 μg/106 cells) (Figure 3A) resulted in the highest recombinant protein yield too (Figure 3B).
The effect of peptones on transfection efficiency and productivity: In the next step, the effect of three different peptones on transfection efficiency and recombinant protein yield were examined. Based on our previous studies 21, the three different media supplements were shown to improve cell growth and productivity in CHO DG44 cells, stably producing chimeric-truncated t-PA. In this research, the transfection medium was supplemented with either Casein Tryptone N1, Soy Peptone A2SC or Soy Peptone E-110 at the time of transfection. All three peptones could improve transfection efficiencies as compared to the optimized transfection from previous steps; they included 59.73, 58.46 and 66.93% transfection efficiency for peptones Tryptone N1, Soy Peptone A2Sc and Soy Peptone E-110 respectively as compared to 51.82% for transfection with no peptone additions (Figure 4A). The effect of peptone addition on recombinant t-PA production was highest with Soy Peptone E-110 (Figure 4B).
As shown in figure 5, the transfection strategy performed in this study could outstandingly increase transfection efficiency of CHO DG44 cell line transfected with pTracer-SV40-mutated t-PA plasmid from 3.6% in starting non-optimized condition to 66.93 % in finally optimized situation.
Discussion :
Over the last decade, TGE has been a widely used strategy for the rapid production of recombinant proteins in mammalian cells. In spite of different studies done to improve this technology in terms of scalability and productivity 22, optimal TGE production yields generally remain lower as compared to values achieved with stable cell lines 23.
Considering the important role of CHO cells as the most common host for therapeutic protein production, it is preferred to have an optimized TGE strategy for the generation of sufficient amount of recombinant protein for pre-clinical investigations. In this way, it is not necessary for biopharmaceutical companies to switch their pre-clinical source cell line of recombinant protein to another stably transfected cell line for final product.
Transfection efficiency is one of the critical elements affecting TGE results. Optimizing the amount of PEI transfection reagent, starting cell densities, DNA plasmid has been shown to have positive effects on transfection efficiencies. However, mostly the optimization studies have been performed on HEK cell line (with higher rates of transfection) and the few studies on CHO cell line have not yet been successful in achieving values as high as HEK cells. Furthermore, the optimized condition also depends on the size and characteristics of DNA plasmid and its compatibility with the cell line, thereby it is important to establish an optimized TGE strategy for each target recombinant protein.
The high cell density used during transfection increases the probability of cell exposures to PEI-DNA polyplexes, and in the hypothermic conditions of culture, the cells would persist in nutrient depletions since they are blocked in a G1 phase with less demand for nutrients 5.
Peptones are widely used as supplements for serum-free culture media. The growth-promoting activity of peptones may have a dual effect in batch cultures. While it may promote rapid cell growth in the first days of culture, it may lead to early depletion of vital nutrients and concomitant release of toxic metabolites, more rapid decline of culture viability, apoptosis and release of proteases which may degrade the product 3. Although the molecular mechanisms of the growth promoting effects of peptones are not fully understood, the positive effects observed could be considered as a consequence of the diverse amino acids composition of the peptones 14,24-26. Despite the fact that a lot of studies have been devoted to investigate the effects of peptones on stable cell line’s production level and growth profile, to our best knowledge, no study has yet been done to see the effect of these elements on transfection efficiency in a TGE process.
In this study, it was proposed that peptone supplementation is a promising approach for TGE optimization. The standard protocol was started with 2×106 cells/ml starting cell density, 0.625 μg/106 cells, 2.5 μg/106 cells which stands for 1:4 DNA to PEI ratio with 3.6% transfection efficiencies. Nevertheless, the three step optimization process for PEI, cell density and DNA concentrations resulted in optimized values of 1.5 μg/106 cells of PEI concentration, 0.5 μg/106 cells of DNA plasmid concentration and cell densities of 0.5×106 cells which finally led to 52.96% transfection efficiency. Therefore, the optimized DNA: PEI ratio was finally set to 1:3. Further optimization done in this research could even increase the transfection efficiency up to 66.93% with the best peptone, Soy peptone E-110. The highest transfection efficiencies with pTracer-SV40-mutated t-PA plasmid of up to 66.93% are comparable with other published data (60-70%) 27.
On the other hand, in terms of day 9 volumetric productivity, the first three steps of optimization (PEI, cell density and DNA concentration) didn’t show significant differences in production of titers; however, in all these three cases, the highest production level was related to the highest transfection efficacy. This may be related to a saturation condition in cell productivity, i.e. higher exposure to plasmid DNA could not impose further expression. The transfection efficiency in mammalian cells such as CHO is one of the elements which can pave the way for higher levels of protein production; however, the level of protein production from heterologous genes introduced into mammalian cells depends upon multiple factors including transfection efficacy, mRNA processing, transportation and stability, the efficacy of translational system, and protein processing. There are different rate limiting steps for efficient gene expression as for each individual protein gene. Therefore, the level of expression does not necessarily correlate with transfection efficacy. This is probably the reason for not having sharply different values of protein production while the transfection efficacy is drastically affected by PEI amount but this effect is not seen in protein production. The final step of optimization, however, was successfully capable of increasing production level along with transfection efficiencies. With peptone feeding strategies, 37% increase in vulometric productivity was achieved (up to 0.861 IU/ml). This can be explained by the positive growth and biomass promoting effect of these peptones explained in our previous studies 20 which maintains the cells in a desirable situation for the optimum transfection. Adding peptones at the same time with DNA-PEI polyplex, supposedly makes larger cells (higher biomass) 21 and with better exposure to the polyplex results in higher transfection efficiencies. Also, growth promoting effect of these peptones could induce recombinant protein production. These yields were achieved from a 9-day batch process and based on our previous experiments, the maximum cumulative titers were achieved at this time 18-20.
One of the promising points in the final optimized strategy was the decrease in the amount of DNA by 25% as compared to un-optimized process. This could also be seen as an important fact for reducing the cost of transfection since plasmid DNA is one of the most expensive components in TGE bioprocesses. Moreover, the PEI transfection reagent was used 66% less than the pre-optimized method which is also helpful for reducing its toxic effects on cell viability. It is expected that the peptone feeding approach utilized in this study would improve TGE yields in CHO cells and this method will be an attractive alternative to TGE in HEK-293 cells for the generation of recombinant proteins in pre-clinical research.
Conclusion :
Despite the improved TGE yields described in the paper, it is also worth mentioning that regular passaging of cells cultivation and fresh handling of PEI stock solution could play an important role in achieving high transfection efficiencies and recombinant protein titers. Moreover, there are still bottlenecks such as the need for media exchange during transfection or cell concentration before transfection which are essential to be considered in order to make it a feasible upscale approach 4,28.
Acknowledgement :
This paper was supported by a grant from the Pasteur Institute of Iran.
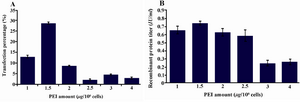
Figure 1. Optimization of PEI amount. Varying amounts of PEI were added as indicated with a constant DNA amount of 1.25 μg/106 cells and a starting cell density of 2×106 cells/ml. A) GFP positive cells 48 hr posttransfection with different PEI concentrations; B)
t-PA recombinant protein concentration in the culture media measured on day 9 posttransfection by ELISA
|
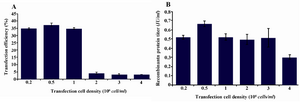
Figure 2. Optimization of the starting cell density for transfection. Varying starting cell densities from 0.2 to 4×106 cells/ml with constant DNA and PEI concentrations were investigated. A) GFP positive cells 48 hr posttransfection with different starting cell densities; B) t-PA recombinant protein concentration in the culture media measured on day 9 posttransfection by ELISA
|
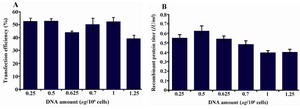
Figure 3. Optimization of DNA concentration for transfection. Varying DNA concentrations from 0.25 to 1.25 μg/106 cells with 0.5×106 starting cell densities and 1.5 μg/106 cells of PEI concentrations were investigated. A) GFP positive cells 48 hr posttransfection with different DNA plasmid concentrations; B) t-PA recombinant protein concentration in the culture media measured on day 9 posttransfection by ELISA
|
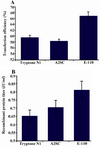
Figure 4. Effect of three different peptones on transfection efficiency and production yield. 1 g/l of final concentrations of peptones were added to the transfection reaction with 0.5 μg/106 cells of DNA, 0.5×106 starting cell density and 1.5 μg/ 106 cells of PEI concentrations. A) GFP positive cells 48 hr posttransfection with different peptone feeding strategies; B) t-PA recombinant protein concentration in the culture media measured on day 9 posttransfection by ELISA
|
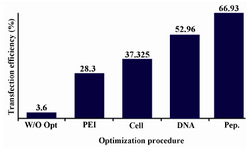
Figure 5. Effect of stepwise optimization on transfection efficiencies of CHO DG44 cells transiently transfected with pTracer-SV40-mutated t-PA plasmid. The optimized values for each step were used in the next experiment
|
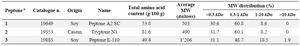
Table 1. Total amino acids content, average molecular weight (MW) and MW distribution of the peptones evaluated in this study
a) Data available from Organotechnie (www.organotechnie.com)
|
|