Periplasmic Expression of a Novel Human Bone Morphogenetic Protein-7 Mutant in Escherichia coli
-
Nematollahi, Leila
-
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
-
 Khalaj, Vahid
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran, +98 21 66480780; v_khalaj@yahoo.com
Khalaj, Vahid
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran, +98 21 66480780; v_khalaj@yahoo.com
-
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
-
Rahimpour, Azam
-
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
-
Jahandar, Hoda
-
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
-
Davami, Fatemeh
-
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
-
 Mahboudi, Fereidoun
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran, +98 21 66480780; mahboudi.f@gmail.com
Mahboudi, Fereidoun
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran, +98 21 66480780; mahboudi.f@gmail.com
-
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
Abstract: Background: Bone Morphogenetic Proteins (BMPs) belong to the transforming growth factor-β (TGF-β) superfamily, and play an important role in bone metabolism. Recombinant forms of BMP-2 and BMP-7 are the only BMPs used clinically. In this study the mature part of human bone morphogenetic protein-7 (BMP-7) was engineered through substitution of the BMP-7 N-terminal sequence by heparin-binding site of BMP-2. This targeted substitution was made to enhance the binding affinity of the novel protein to the extracellular matrix components such as heparin and heparan sulfate proteoglycans (HSPGs).
Methods: The engineered protein was expressed in Escherichia coli (E.coli). The PelB signal sequence was used to translocate soluble pro¬teins into the periplasmic space of E.coli. The protein was purified from periplasmic extract using Ni-NTA chromatography and the SDS-PAGE and western blot analysis confirmed the successful expression of the novel protein.
Results: The novel hBMP-7 mutant was produced as approximately 16 kDa monomer. It was found that the heparin binding of this protein was approximately 50% more than that of the wild-type at a protein concentration of 500 ng/ml.
Conclusion: The findings showed that the periplasmic expression may be suitable to produce complex proteins like BMPs.
Introduction :
Bone morphogenetic protein-7 (BMP-7; also called osteogenic protein-1), is a member of the Transforming Growth Factor b (TGF-b) superfamily, which was originally isolated from bone (1-3).
Studies have shown that human BMP7 generated by recombinant DNA technology can induce ectopic cartilage and bone formation (1,3,4). Due to the short half-life of BMP-7, high doses are required to induce bone healing. High doses are not only costly, they cause undesirable side effects such as ectopic bone formation (5-7). To overcome the difficulties in using BMPs as therapeutic agents, some strategies have been developed. One of them is genetic engineering of BMPs to increase binding capacity to the extracellular matrix components such as heparin and heparan sulfate proteoglycans (HSPGs) (8). These macromolecules have binding affinity with a number of growth factors such as BMP-2 and can regulate its biological activity (9,10). This interaction with glycosaminoglycan allows the formation of localized gradients by inhibiting the diffusion of these biologically important proteins (11). This may decrease the required dose of the morphogen due to its prolonged local retention time at application site (12,13).
Another approach is expression of rhBMP-7 in different expression systems to achieve large scale production (1,2). So far, BMP-7 has been expressed both in eukaryotic and prokaryotic cell culture systems (1,2,4). Mammalian systems are capable of producing biologically active proteins. However, the production of the recombinant human Bone Morphogenetic Proteins (rhBMPs) from the mammalian cell cultures has a low production yield and high cost. Prokaryotic systems (E.coli) were also used for the production of BMPs. Although cytoplasmic expression allows the production of high level of recombinant proteins, it suffers from several disadvantages. Often overexpressed proteins accumulate in inclusion bodies and the complex refolding processes are necessary to obtain active proteins. The secretion of recombinant proteins to the E.coli periplasmic space may eliminate these drawbacks (14-16).
Periplasmic expression has several advantages over the cytosolic one as follows: i) it simplifies protein purification, ii) it maintains an oxidizing environment to promote proper protein folding, removal of signal peptide and elimination of polypeptides, iii) the outer membrane can be selectively degraded by simple osmotic shock, iv) the periplasm is accessible to molecules, e.g. ligands, in order to enhance stability and folding of recombinant proteins (15-18).
In the case of BMP-7, genetic engineering approach to produce more potent form of this protein with intensified connection to the extracellular matrix components has not been considered. Taking advantage of periplasmic expression and genetic engineering, we developed an alternative platform to produce the novel protein in E.coli. The novel protein was named B2BMP-7 (B2 denoting the heparin-binding site of BMP-2). In B2BMP-7, the first 16 N-terminal amino acids of BMP-7 mature part have been replaced by BMP-2 heparin-binding site in order to increase heparin affinity.
Materials and Methods :
E.coli strains Top10 F' (Novagen) was used as the host for recombinant plasmid. BL21 (DE3) (Novagen, USA) was applied as expression host. pET-22b (Novagen, USA) was utilized as expression vector. Bacterial strains were grown in LB agar and LB Broth. Rabbit polyclonal antibody to BMP-7 was obtained from Abcam (Cambridge, UK) and goat anti rabbit IgG-HRP conjugated was purchased from Razi BioTech (Tehran, Iran). All reagents for SDS/polyacrylamide gel electrophoresis were from Bio-Rad, (CA, USA). Plasmids and DNA fragments were purified using a miniprep kit (QIAGEN, USA) and a gel extraction kit (QIAGEN, USA).
Preparation of the novel construct: A novel construct containing the cDNA sequence encoding genetically modified mature domain of BMP-7 (139 amino acids) was synthesized by GeneRayBiotech (Shanghai, China). As shown in figure 1, NdeІ (5´…CA TATG … 3´ ) and EcoRІ (5´… GAATTC … 3´ ) restriction sites were inserted in the upstream and downstream of gene respectively to facilitate sub-cloning into the expression vector. pelB and His–Tag coding sequences were introduced to the N-terminal and C-terminal of the construct respectively. pelB signal sequence is necessary for potential periplasmic localization. In genetically modified mature domain, the first 16 N-terminal amino acids of BMP-7 (STGSKQRS QNRSKTPK) were substituted by the first 16 amino acids of BMP-2 without cysteine 16 (Q AKHKQRKRLKSSKRH). In order to prevent the formation of undesirable intrapolypeptide disulfide bound, the residue 16 (cysteine) of BMP-2 heparin-binding site was deleted.
Cloning of the novel construct: To prepare the final expression construct, the synthetic gene was cut from an intermediate vector, pGH, using NdeІ and EcoRІ enzymes and was subsequently cloned into the multiple cloning site of pET-22b. The recombinant plasmid, pET_B2BMP7, was confirm-ed by the restriction-enzyme analysis using NotI/EcoRV enzymes.
Transformation and induction of protein synthesis: The transformed E.coli BL21(DE3) strain with recombinant plasmid were cultured in LB medium containing ampicillin and incubated at 37°C until an OD 600 of 0.5. The induction was carried out by adding IPTG at a final concentration of 1 mM. The cells were collected by centrifugation at appropriate times (2 and 4 hr) post-induction. A part of the culture was used as negative control without adding IPTG (pre-induction).
Preparation of periplasmic protein: Periplasmic osmotic shock was carried out as described previously (19). Briefly, the pellet was re-suspended in 80 ml/g cells of TES buffer (Tris-Hcl 30 mM, EDTA 1 mM, Sucrose 20%, pH=8.0). The mixture was then incubated on ice for 5-10 min with gentle agitation. The cells were then centrifuged at 8000xg for 20 min. The supernatant was removed and the pellet was re-suspended in the same volume of ice-cold 5 mM MgSO4. After shaking for 10 min in an ice bath, the mixture was centrifuged at 8000 x g for 20 min and the supernatant was collected for periplasmic protein. The periplasmic proteins were dialyzed overnight against lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole pH ~8.0). Purification was then performed under native conditions by Ni-NTA resin as described by manufacturer (QIAGEN, USA). The periplasmic extract was applied to a column containing Ni-NTA resin equilibrated with lysis buffer. After elution by elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole pH=8.0), the fraction containing the novel protein was pooled and concentrated by Microcon filtering system (Millipore, USA).
SDS- PAGE and western blot analysis: SDS–PAGE was carried out in a 15% resolving polyacrylamide gel according to the Laemmli method (20). For western blot, the samples were separated using SDS-PAGE and electro-transferred to a nitrocellulose membrane, blocked with 5% non-fat dry milk, incubated with 1:1000 dilution of rabbit anti-human BMP-7 (Abcam, Cambridge, UK)
(90 min) followed by peroxidase conjugated goat anti-rabbit antibody (Razi Biotech, Iran) (1:1000) for 45 min. The antigen-antibody complex was then visualized by DAB-HRP or ECL system. Immunoblotting was carried out on the purified periplasmic fraction using Anti- His_6 -Peroxidase (Roche, Germany). The ECL detection system (GE Healthcare) was utilized for chemiluminescence development. Total protein concentration was analyzed by the Lowry method (21) using bovine serum albumin as the standard.
Alkaline phosphates release assay: To assess the biological activity of B2 BMP-7, C2C12 cells were grown in DMEM containing 10% FBS and penicillin/ streptomycin at 37°C, 5% CO2. For the Alkaline Phosphates (ALP) release assay, 100,000 cells/well were incubated in a 24-well plate for 24 hr, after which the supernatants were removed, and 1 ml of fresh medium containing different concentrations of the purified B2BMP-7 protein (0, 500, 1000 ng/ml) was added. Different concentrations of rhBMP-7 from CHO (0 and 500 ng/ml) (ProSpec, NJ, USA) and rhBMP-7-His from E.coli (0, 500, 1000 ng/ml) (ProSpec, NJ, USA) were used as controls. After 3 days incubation, the cells were rinsed with PBS and lysed by 0.2% Triton X-100 for 10 min at 4°C. The cell lysates were then centrifuged for 10 min at 2500 r.c.f., and the supernatants were used for the enzyme assay. ALP release assay was performed using a commercial assay kit (Sensolyte ® рNPP Alkaline Phosphatase Assay Kit from AnaSpec) according to the manufacturer’s protocol. After incubation of clarified supernatant with p-nitrophenyl phosphate substrate solution for 30 min at 37°C, the reaction was stopped and optical density at 405 nm was measured with an ELISA reader.
Heparin binding assay: Binding assay, based on those described previously (22), was carried out by BD™ Heparin Binding Plates (BD Biosciences, MA, USA). Briefly, the plates were incubated with heparin sodium salt from porcine intestinal mucosa (H3393, Sigma) overnight at room temperature, using 25 µg/ml of heparin in Phosphate-Buffered Saline (PBS). Plates were washed in PBS and blocked with 1% (w/v) BSA in PBS for 60 min at 37°C. Wells were rewashed and incubated with different concentrations of rhBMP-7-His and purified B2 BMP-7 protein (0, 250, 500, 1000 ng/ml) at 37°C for 2 hr.
As a negative control, the proteins also bind to the non-heparin coated wells. The interaction was assessed by adding 100 µl/well of a 1:1000 dilution of rabbit anti-human BMP-7 antibody (Abcam, Cambridge, UK) (90 min) followed by 100 µl/well of peroxidase conjugated goat anti-rabbit antibody (Razi Biotech, Iran) (1:1000) for an additional 45 min. The absorbance values at 450 nm were measured after 15 min.
Results :
Construction of the expression vector: The fragment encoding the genetically modified mature part was successfully cloned into the NdeІ/EcoRІ site of pET-22b vector (Novagen, USA). The restriction enzyme analysis using NotI/EcoRV enzymes showed two fragments with expected sizes at 4095 and 1820 bp (Figure 2).
Expression analysis: Following induction, total protein of induced and non-induced cells was compared on a SDS-polyacrylamide gel (Figure 3A). The bands related to B2BMP-7 expressed by BL21 (DE3) transformant is shown by the arrows. The protein was reactive to rabbit-anti-human BMP-7 antibody (Abcam, USA) as evidenced by western blot analysis (Figure 3B). The molecular weight of the recombinant protein is in accordance with the calculated molecular weight of B2BMP-7 pre-protein (pelB-hB2BMP-7) (~18 kDa). Note that the B2BMP-7 represents approximately 37% of the total cell protein according to the densitometry analysis.
Periplasmic expression analysis: Periplasmic extract and cell-pellet fraction were analyzed by SDS–PAGE using silver staining. In figure 4A an over-expression of unprocessed pre-protein was detected in cell pellet fraction. The processed mature protein was mostly detected in the periplasmic extract. The purified periplasmic protein appeared as a single ~16 kDa band as evidenced by western blot analysis (Figure 4C).
Biological activity test: To assess the osteogenic activity of novel protein, alkaline phosphatase release assay was performed. The purified monomer did not show any significant biological activity. No enzyme activity was similarly detected in the C2C12 cultures by commercial hBMP-7-His from E.coli (ProSpec, NJ, USA). Commercial hBMP-7 from CHO cells (ProSpec, NJ, USA) was used as positive control and stimulated alkaline phosphatase release in a dose-dependent manner (Figure 5).
Heparin binding assay: BD™ Heparin Binding Plates were used in heparin binding assay. The results showed that both commercial hBMP-7-His and its mutant bind to heparin in a dose-dependent manner and the background binding related to the non-heparin coated wells was considerably lower. The heparin binding of the B2 BMP-7 was approximately 50% higher than that of the wild-type at a protein concentration of 500 ng/ml (Figure 6).
Discussion :
Several studies have shown that the natural activity and distribution of BMPs can potentially be modulated by glycosaminoglycans (GAGs) as part of the ECM (23). One approach to take advantage of this issue is modification of BMPs to enhance connection to the extracellular matrix components. For example, modification of heparin-binding site of BMP-2 has shown a significant increase in its local retention time and various mutants of BMP-2 with higher osteoinductivity have been produced (12,13).
Recombinant human BMP-7 (OP-1) has been approved for treatment of long bone nonunions and spinal fusion (24,25). Due to the high cost of this protein, the availability of BMP-7 for treatment is limited (1). Therefore, in this study for the first time we used two strategies simultaneously to produce the novel BMP-7 protein with improved properties in E.coli. One of them is genetic engineering of BMP-7 to increase binding capacity to heparin. Novel BMP-7 mutant was engineered by substitution of the N-terminal of BMP-7 mature part by the BMP-2 heparin-binding site. The heparin-binding site of BMP-2, which contains multiple basic triplets, participates in binding to the components of the extracellular matrix (26). It seems that addition of these basic triplets (e.g. Arg -Lys -Arg, RKR) could be an advantage.
It is reasonable to propose that B2BMP-7 with extra basic residues at its N-terminal may possess higher heparin affinity compared to the commercial form. To test this hypothesis, the binding of rhBMP-7-His and its mutant to heparin was investigated by an ELISA assay using BD™ Heparin Binding Plate. It was found that both commercial BMP-7-His and its mutant bind to heparin in a dose-dependent manner. Furthermore, in accordance with our hypothesis the heparin affinity was approximately 50% more than that of the commercial form at a protein concentration of 500 ng/ml. Despite the production of novel protein as inactive monomeric form, the improved heparin binding, up to 50%, is greatly promising. As it was mentioned BMP-2 mutants with intensified connection to heparin possess significantly higher biological activity in vivo (12,13). In spite of the fact that novel protein with higher affinity to heparin may possess increased osteogenic activity in vivo, this issue has to be clarified by further examinations.
So far, rhBMP-7 has been produced in E.coli using a pET expression system (3). However, disadvantages of cytoplasmic expression led us to develop an alternative strategy to produce novel BMP-7 protein using periplasmic expression.
Initially, B2BMP-7 was produced in the form of inclusion bodies representing 37% of the total cell protein. As shown in figure 3A, the novel protein is the main protein in the cell lysate and the lack of expression in the pre-induction lane shows a tight regulation of expression in pET22b vector. In this system, the signal sequence, pelB, is responsible for the secretion of protein into the periplasmic space. In the cell pellet fraction the prominently expressed protein was the B2BMP-7 pre-protein (pelB-hB2BMP-7). However, in the periplasmic extract there was detectable amount of mature hBMP-7. The periplasmic content was released from the bacterial pellet by osmotic shock. In this study, the protein was obtained in its soluble form and purification was carried out easily in a single step by Ni-NTA chromatography. The results of SDS-PAGE and western blot analysis confirmed the successful secretion of the processed protein into the periplasm.
Transfection of the full length cDNA of human BMPs has been performed in different mammalian expression systems such as CHO, BSC-1 (27), and COS7 (28) cells. However, proteolytic processing events create a heterogeneous mixture of BMPs (29). In contrast to the mammalian expression systems, the amino-terminal heterogeneity of the protein was limited by expression of the mature part. As a result, a homologous mixture of the processed protein was produced in the present study.
The biological activity of new protein was tested by the induction of alkaline phosphatase activity in C2C12 cells. The monomeric form that was obtained in this stage did not show any biological activity similar to the commercial BMP-7-His from E.coli (Pro Spec). The result is in agreement with previous findings in which the biological active hBMP-7 was described as homodimeric or heterodimeric forms (3, 30,31).
In the periplasmic expression several factors are involved in the processing efficiency such as signal sequence, host strain, expression level, growth and inducing conditions (32). It seems that in the case of complex disulfide-bonded proteins like BMPs, further optimization is required to obtain a fully active protein.
Conclusion :
This is the first report describing periplasmic expression of a novel hBMP-7 mutant in E.coli. The novel protein showed a significantly higher heparin affinity compared to the wild type. As the functionality of BM P-7 is highly dependent on its proper folding and disulfide bond formation, future optimization of the periplasmic expression may result in the production of the fully active dimer protein.
Acknowledgement :
The authors would like to thank Mrs. Farzaneh Barkhordary for her valuable support during this project. This study was financially supported by Pasteur Institute of Iran.

Figure 1. Schematic representation of designed cassette gene. His -Tag coding sequence was engineered to the C-terminal of the construct in order to facilitate purification using Ni-NTA technology
|
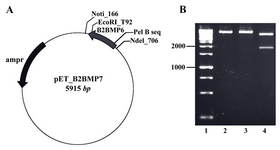
Figure 2. A) The map of recombinant plasmid (pET_ B2BMP7). The size of expression construct was 5915 bp, B) Restriction analysis of recombinant plasmid. Lane 1: Size marker; lanes 2, 3: Digestion of pET_B2BMP7 with NotI or EcoRV created linear plasmids (5915 kb); lane 4: Digestion of recombinant plasmid with NotI/EcoRV created two fragments with expected sizes at 4095 bp and 1820 bp
|
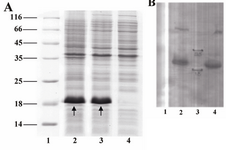
Figure 3. A) SDS-PAGE analysis of a recombinant clone producing novel mutant. Lane 1, Protein marker SM0671; lanes 2, 3, two and four hours samples (post induction); lane 4, pre-induction sample. Arrows indicate the bands related to the pre-protein (pelB-hB2BMP-7). B) Western blot analysis of recombinant protein. Lane 1, pre-induction sample; lanes 2, 4, two and four hours samples (post induction); lane 3, Protein marker
|
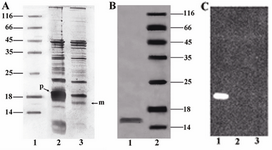
Figure 4. A) SDS-PAGE analysis of the periplasmic extract and cell-pellet fraction. Unprocessed pre-protein (~18 kDa) and processed mature protein (~16 kDa) are indicated as (p) and (m) respectively. Lane 1, Protein marker SM0671; lane 2, Cell-pellet fraction; lane 3, Periplasmic extract, B) SDS-PAGE analysis of the purified protein. Lane 1, Concentrated purified mutant; lane 2, Protein marker, C) Western blot analysis of the mutant, Lane 1: purified mutant; lane 2: Protein marker; lane 3:pre-induction sample as negative control
|
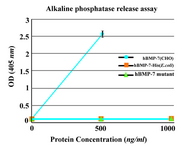
Figure 5. Alkaline phosphatase release assay. The purified monomer did not show any significant biological activity similar to the commercial BMP-7-His. As positive control, commercially available rhBMP-7 produced in CHO cells was used. Each value is the mean of triplicates shown±SD
|
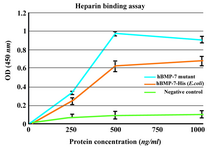
Figure 6. Heparin binding assay of BMP-7-His and B2BMP-7. The results showed dose-dependent binding of commercial BMP-7-His and its mutant to heparin. The background binding related to the non-heparin coated wells as negative control was considerably lower. Each absorbance value is the mean of triplicates shown±SD
|
|