Differentiation of Bovine Spermatogonial Stem Cells into Osteoblasts
-
Qasemi-Panahi, Babak
-
Department of Clinical Science, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
-
 Tajik, Parviz
D.V.M., D.V.Sc., Stem Cell Research Center, Veterinary Faculty, University of Tehran, Tel: +98 21 66929532 Fax: +98 21 66933222 E-mail: ptajik@ut.ac.ir
Tajik, Parviz
D.V.M., D.V.Sc., Stem Cell Research Center, Veterinary Faculty, University of Tehran, Tel: +98 21 66929532 Fax: +98 21 66933222 E-mail: ptajik@ut.ac.ir
-
Department of Clinical Science, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
-
Movahedian, Mansooreh
-
Department of Anatomy, Faculty of Medical Science, University of Tarbiat Modares, Tehran, Iran
-
Moghaddam, Gholamali
-
Department of Animal Science, Faculty of Agriculture, University of Tabriz, Tabriz, Iran
-
Barzgar, Younes
-
Department of Clinical Science, Faculty of Veterinary Medicine, University of Tabriz, Tabriz, Iran
-
Heidari-Vala, Hamed
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
Abstract: Spermatogonial Stem Cell (SSC) technologies provide multiple opportunities for research in the field of biotechnology and regenerative medicine. The therapeutic use of Embryonic Stem Cells (ESCs) is restricted due to severe ethical and immunological concerns. Therefore, we need a new pluripotent cell type. Despite well-known role of germ cells in the gametogenesis, some facts apparently show their multipotentiality. In the present study, bovine SSCs were co-cultured with Sertoli cell for 7 days. Sertoli cells and SSCs were identified by Vimentin and Oct-4 immunocytochemical staining method, respectively. In order to differentiate SSCs into osteoblasts, we used consecutive inducer media without separation of the colonies. We characterized osteoblasts using Alizarin red staining.
Introduction :
Nowadays, regenerative medicine is one of the pioneer fields of science including bioengineering, cell biology, molecular biology and surgery (1-3). Since Embryonic Stem Cells (ESCs) have more accessibility and the appropriate potential to differentiate into different lineages, majority of current studies are based on ESCs (4,5).
ESCs are the well-known pluripotent stem cells by now, as they have the capacity of self-renewal and differentiation into different cell types (6). However, severe ethical, legal and immunological considerations have restricted the ESCs indication. In order to resolve these concerns, we need new pluripotent cell types that have the potential to differentiate into various cell types.
According to Streckfuss-Bomeke et al, Germ-line Stem Cells (GSCs) are capable of self-renewal and differentiating into all germ layer derivatives. Recently, some studies show that SSCs can be applied to generate pluripotent stem cells without genetic manipulation. In addition they can be used for autologous transplantation without ethical and immunological problems. These findings suggest that human SSCs may have considerable potential for cell/regenerative therapy (7). Even with these properties, GSCs can be a potential candidate of cell source for regenerative-therapy in neurodegenerative diseases such as Alzheimer (8).
Thus, SSCs can be preferred as a proper source for regenerative studies. Also, the pluripotency of GSCs was confirmed by in vitro differentiation toward hepatic and neuronal lineages and formation of embryonic chimeras after injection into blastocysts (9). In this study, we show that SSCs are able to differentiate into Osteoblasts in vitro.
Materials and Methods :
Animal
Three to 4 months old male calves from the Aminabad Research Institute, University of Tehran (Tehran, Iran), were used.
Germ cell collection
Testicular germ cells collection was done with enzymatic digestion according Izadyar et al with a little modification (10). Briefly, obtained testis pieces were mechanically minced and floated in DMEM containing 1 mg/ml collagenase, 1 mg/ml Trypsin, 1 mg/ml hyaluronidase type II and 5 μg/ml DNase I and then incubated at 32C for 60 min. After three time washing in DMEM and excluding the interstitial cells, for secondary digestion step, seminiferous tubules were incubated in DMEM containing collagenase, hyaluronidase and DNase for 45 min. Finally, obtained cellular suspension was centrifuged at 30 g for 2 min to achieve favorite cell population. Then, spermatogonial cells were co-cultured with Sertoli cell for 7 days.
Sertoli cell collection
A little solution of Datura stramonium agglutinin lectin (DSA; Sigma) 5 µg/ml in TBS was poured into the sterile flasks. After 1 hr when the dishes were coated well with DSA-Lection, dishes were washed three times with DMEM containing 0.5% BSA. Cell solution obtained from enzymatic digestion was added to DSA-lectin coated flasks and incubated at 32 C for 1 hr in a humidified atmosphere with 5% CO2. After 4 days when the Sertoli cells were constructed as a monolayer with a proper confluency, they were resuspended with EDTA-trypsin treatment (0.02% EDTA- 0.1% trypsin in PBS) at 37 C for 5 min. Finally, Sertoli cell population were distributed in favorite volumes and underwent secondary culture in DMEM.
Immunocytochemistry for SSCs and Sertoli cell identification
Vimentin was detected in Sertoli cell, by the procedure which was described by Tajik et al (11). Briefly, anti-vimentin (Abcam, Cambridge, UK) optimally diluted in TBS/BSA (5 μg/ml and 2 μg/ml, respectively) was applied over slides for 60 min at room temperature. After being washed as above, Fluorescein Isothiocyanate (FITC)-conjugated sheep anti-mouse Ig was diluted in TBS/BSA in a ratio of 1:50 and incubation was further continued for 45 min at room temperature. Following once washing with TBS/BSA, slide was exposed to 7-Amino- actinomycin D (7AAD) 5 μg/ml for 5 min. It was then washed and mounted in PBS-glycerol 90% and examined under a fluorescence microscope (Olympus, Tokyo, Japan).
Oct-4 has been described as a marker for undifferentiated cells (12). The obtained colonies of SSCs were immuno cytochemically stained with anti-Oct4 (conjugated with FITC). Briefly, anti-Oct4 (Abcam) diluted in TBS/BSA, was applied over slides for 60 min at room temperature. After washing, FITC-conjugated donkey polyclonal secondary antibody to Goat IgG was added and incubation was further continued for 45 min at room temperature. After washing with TBS/BSA, it was mounted in PBS-glycerol 90%, and examined under a fluorescence microscope (Olympus, Tokyo, Japan).
Differentiation into osteoblast
After 1 week, the culture medium of experimental group was exchanged with induction medium. But in control group, previous culture was continued. The induction medium included DMEM high glucose with 10% FBS, 2 mM L- Glutamin, 100 IU/mL penicillin, 100 µg/mL streptomycin, 50 µg/ml ascorbate-2-phosphate, 5 mol ß- glycerol phosphate and 10 nM Dexamethasone (13,14). The cultures were refreshed twice a week.
Osteoblast cells identification
After 21 days and in order to confirm osteoblastic differentiation, cells fixed with formaldehyde 40% were incubated for 10 min at room temperature in a 1 ml of 40 mM Alizarin Red (sigma) solution pH=4. Finally, slides were washed with TBS/BSA and then exposed to light microscope.
Results :
The Sertoli cell population obtained from DSA-lectin isolation, proliferated and created a monolayer of cells (Figure 1A). The Sertoli cells had 80% confluency and Vimentin was detected in this feeder monolayer cells (Figure 2A). The colonies appeared four days after co-culture. We didn’t separate colonies, but culture media was replaced with induction media. The morphology of a spermatogonial-derived colony on monolayer Sertoli cell is shown in figure 1B.
In immunocytochemical staining, expression of Oct-4 was found in undifferentiated spermatogonial stem cells (Figure 2B), but were not expressed in the cells differentiated in osteogenic medium. Using Alizarin red staining, the cultured cells were stained on day 21 for assessing the mineralized cells. Alizarin red staining appeared as microscopic observation of osteoblasts differentiated from spermatogonial cells (Figure 2C). Also figure 2D showed the control SSCs culture while induction medium was not added to it.
Discussion :
This study describes osteoblastic differentiation of bovine Spermatogonial Stem Cells (SSCs). The differentiation of SSCs for tissue regeneration is a promising alternative to adult stem cells. Ethical and immunological problems cause a range of limits in ESCs using as a reliable cellular source for therapeutic aims. The current study demonstrated alternative cell source for regenerative medicine.
In spite of germ cells specificity in gametogenesis, some evidences have indicated their multipotentiality. For example, teratoma tumors which contain some cell types and tissues in various maturation states, were seen exclusively in gonads (15). Furthermore Guan et al showed that germ-line stem cells obtained from neonatal mouse testis are pluripotent. They suggested that the germ-line lineage may be able to generate pluripotent cells (16). Cells expressing Oct-4 can differentiate into pluripotent cells (17,18).
As shown in figure 2B, bovine spermatogonial stem cells can express Oct-4, so we can conclude that these cells have pluripotency characteristic. In this study, we differentiated bovine spermatogonial stem cells into osteoblasts. They play an important role in bone formation and homeostasis having close cooperation with osteoclasts (19). Osteoblast differentiation of embryonic stem cells (19, 20) and mesenchymal stem cells (21) have been performed in the past. Alizarin red staining was used to observe the mineralization (22). Recently, some studies reported that chicken spermatogonial stem cells differentiate into various cell such as osteoblast (23), while reports of SSCs differentiation into osteoblast in mammalian have not been reported yet. It appears that the current study is the first report, that shows osteoblastic differentiation of bovine SSCs.
Conclusion :
In conclusion, the results of present investigation demonstrated that establishment of Germ-line Stem Cells (GSCs) from human testicular biopsies may allow stem cell therapy strategy with less immunological and ethical concerns than embryonic stem cells.
Acknowledgement :
We are very grateful for the collaboration of Avicenna Research Institute, especially Dr Amir Hassan Zarnani. In addition, we thank Stem Cell Research Center of Veterinary Faculty, University of Tehran. We are very grateful for Mr Kioomars Saliminejad helping in the Art Works.
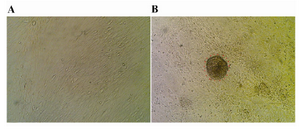
Figure 1. Sertoli and spermatogonial cells: A) Monolayer of bovine Sertoli cell; B) Spermatogonial-derived colony on a monolayer of Sertoli cell; ×200
|
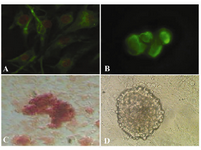
Figure 2. Immunocytochemical and alizarin red staining for cell identification; A) Vimentin was detected in the feeder monolayer cells; B) Oct-4 was detected in cells of colony (before differentiation); C) Alizarin red staining of mineralized cells on day 21 (after differentiation); 400×; D) SSCs culture without induction medium
|
|