Biogenic Silver Nanoparticles by Gelidiella acerosa Extract and their Antifungal Effects
-
Vivek, Marimuthu
-
Department of Biotechnology, School of Life Sciences, Karpagam University, Coimbatore, Tamil Nadu, India
-
Senthil Kumar, Palanisamy
-
Department of Biotechnology, School of Life Sciences, Karpagam University, Coimbatore, Tamil Nadu, India
-
Steffi, Sesurajan
-
Department of Biotechnology, School of Life Sciences, Karpagam University, Coimbatore, Tamil Nadu, India
-
 Sudha, Sellappa
Ph.D., Department of Biotechnology, Karpagam University, Coimbatore, Tamil Nadu, India , Fax: +91-422-2611043 E-mail: sudhasellappa@yahoo.co.in
Sudha, Sellappa
Ph.D., Department of Biotechnology, Karpagam University, Coimbatore, Tamil Nadu, India , Fax: +91-422-2611043 E-mail: sudhasellappa@yahoo.co.in
-
Department of Biotechnology, School of Life Sciences, Karpagam University, Coimbatore, Tamil Nadu, India
Abstract: The synthesis, characterization and application of biologically synthesized nanomaterials are an important aspect in nanotechnology. The present study deals with the synthesis of silver nanoparticles (Ag-NPs) using the aqueous extract of red seaweed Gelidiella acerosa as the reducing agent to study the antifungal activity. The formation of Ag-NPs was confirmed by UV-Visible Spectroscopy, X-Ray Diffraction (XRD) pattern, Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM). The synthesized Ag-NPs was predominately spherical in shape and polydispersed. Fourier Transform Infra-Red (FT-IR) spectroscopy analysis showed that the synthesized nano-Ag was capped with bimolecular compounds which are responsible for reduction of silver ions. The antifungal effects of these nanoparticles were studied against Humicola insolens (MTCC 4520), Fusarium dimerum (MTCC 6583), Mucor indicus (MTCC 3318) and Trichoderma reesei (MTCC 3929). The present study indicates that Ag-NPs have considerable antifungal activity in comparison with standard antifungal drug, and hence further investigation for clinical applications is necessary.
Introduction :
Generally, metal nanoparticles are synthe-sized and stabilized through chemical and mechanical methods (1,2), electrochemical techniques (3), photochemical reactions in re-verse micelles (4) and nowadays via green chemistry method (5). Synthesis of nanopar-ticles through biological method is a good, environment friendly and economically alter-native method. Synthesis of green nanomater-ials and their characterization is an emerging field of nanotechnology from the past few decades, because of their applications in the fields of physics, chemistry, biology and medicine.
Application of green chemistry to the syn-thesis of nanomaterials has vital importance in medicinal and technological aspects (6,7). Biologically synthesized silver nanoparticles (Ag-NPs) have wide range of applications because of their remarkable physical and chemical properties. There is a very little literature on the extra cellular biosynthesis of Ag-NPs using plants and pure compounds from plants (8-10). Specifically, while there is relatively little or no literature on the extra cellular synthesis of Ag-NPs by using sea-weeds (11).
In this article, we describe a simple one-step method for the synthesis of Ag-NPs by the reduction of aqueous Ag-ions using ex-tracts of red sea weed, at direct sunlight condition. The amount of nanoparticles syn-thesized and qualitative differences between synthesized nanoparticles were also investi-gated by various analytical methods.
Gelidiella acerosa (Forsskal) (Class: Flori-deophyceae Order: Gelidiales, Family: Geli-diellaceae, Genus: Gelidiella) is an abundant-ly growing seaweed in Coastal areas of south India. It occurs in inter tidal region of Gulf of Mannar Southeast coast of India. Gelidiella acerosa has been used as gelling agent to make jellies, calorie free cookery ingredient, valuable antioxidant for treating ROS medi-ated diseases and useful post-coital contracep-tive (12).
Present study is the first report on the synthesis of highly stable Ag-NPs using com-monly available marine algae Gelidiella ace-rosa. The results reported here cover the biological synthesis of Ag-NPs and their anti-fungal activity.
Materials and Methods :
Sample collection
In the present study, Gelidiella acerosa (Forsskal), a red seaweed was collected from Mandapam coastal region (78°8’E, 9°17’N), Gulf of Mannar, Tamilnadu, South India. Samples were brought to laboratory in poly-thene bags and cleaned thoroughly with fresh water to remove adhering debris and associ-ated biota. The algae were cleaned using brush for the removal of the epiphytes with distilled water. After cleaning, algae were dried in shade at room temperature for one week.
Extraction
The whole plant of Gelidiella acerosa were initially rinsed thrice in distilled water and dried on paper toweling, and samples (25 g) were cut into fine pieces and boiled with 100 ml of sterile distilled water for 5 min. The crude extract was passed through Whatman No.1 filter paper and the filtrates were stored at 4°C for further use.
Synthesis of Ag-NPs
Silver nitrate (AgNO3) was of analytical grade (AR) and purchased from E. Marck (India). In the typical synthesis of silver nanoparticles, 10 ml of the aqueous extract of Gelidiella acerosa was added to 90 ml of 1 mM aqueous AgNO3 solution in 250 ml conical flask and kept at room temperature for 48 hr at 120 rpm. Suitable controls were maintained throughout the conduct of experi-ments.
UV-Vis spectral analysis
The colour change in reaction mixture (metal ion solution + seaweed extract) was recorded through visual observation. The bio reduction of silver ions in aqueous solution was monitored by periodic sampling of ali-quots (0.5 ml) and subsequently measuring UV-Vis spectra of the solution. UV-Vis spec-tra of these aliquots was monitored as a function of time of reaction on UV-Vis spec-trophotometer UV-2450 (Shimadzu).
XRD analysis
The Ag-NPs solution thus obtained was purified by repeated centrifugation at 5000 rpm for 20 min followed by redispersion of the pellet of Ag-NPs in 10 ml of deionized water. After freeze drying of the purified Ag- NPs, the structure and composition were ana-lyzed by XRD and SEM. The dried mixture of Ag-NPs was collected for the determin-ation of the formation of Ag-NPs by an X’Pert Pro x-ray diffractometer (PAN analyt-icalBV, The Netherlands) operated at a volt-age of 40 kV and a current of 30 mA with Cu Kα radiation in a θ- 2 θ configuration. The crystallite domain size was calculated from the width of the XRD peaks, assuming that they are free from non-uniform strains, using the Scherrer’s formula:
D= 0.94 λ / β Cos θ
1) where D is the average crystallite do-main size perpendicular to the reflecting planes, λ is the X-ray wavelength, β is the full width at half maximum (FWHM), and θ is the diffraction angle. To eliminate additional in-strumental broadening the FWHM was cor-rected, using the FWHM from a large grained Si sample:
β corrected = (FWHM2sample- FWHM2si)1/2
2) This modified formula is valid only when the crystallite size is smaller than
100 nm (13).
SEM analysis
Scanning Electron Microscopic (SEM) an-alysis was done using Hitachi S-4500 SEM machine. Thin films of the sample were pre-pared on a carbon coated copper grid by just dropping a very small amount of the sample on the grid, extra solution was removed using a blotting paper and then the film on the SEM grid were allowed to dry by putting it under a mercury lamp for 5 min.
TEM analysis
The structural characterization of the silver nanoparticles was carried out by Transmission Electron Microscopy (TEM). The sample was prepared by air-drying drops of diluted solu-tions of the preparations on carbon films sup-ported by copper grids.
FTIR analysis
To remove any free biomass residue or compound that is not the capping ligand of the nanoparticles, the residual solution of 100 ml after reaction was centrifuged at 5000 rpm for 10 min.
The supernatant was again centrifuged at 10000 rpm for 60 min and the pellet was obtained .This is followed by redispersion of the pellet of Ag-NPs into 1 ml of deionized water. Thereafter, the purified suspension was freeze dried to obtain dried powder. Finally, the dried nanoparticles were analyzed by FTIR Nicolet Avatar 660 (Nicolet, USA).
Antifungal activity
The antifungal activity of Ag-NPs was evaluated against the following human patho-genic strains: Humicola insolens (MTCC 4520), Fusarium dimerum (MTCC 6583), Mucor indicus (MTCC 3318) and Tricho-derma reesei (MTCC 3929). These fungal strains were obtained from the Institute of Microbial Technology, Chandigarh, India. Cultures were maintained on potato dextrose agar (Hi Media, India) slants and they were subcultured before use. The fungal strains studied were clinically important, causing several infections and it is essential to over-come them through some active therapeutic agents.
The antifungal assay was performed by agar well diffusion method; a well was pre-pared in the plates with the help of cork-borer (0.85 cm). Using sterile micropipette, 50 μl (5 mg/ml) of the sample of nanoparticle solution was loaded in three plates along with positive control (containing 5 mg/ml Clotri-mazol). After incubation at 37C for 48 hr, the different levels of zone of inhibition were measured using the Hi antibiotic zone scale.
Results :
It is well known that Ag-NPs exhibit reddish-brown in water (14). The formation of Ag-NPs by reduction of the aqueous Ag+ during exposure to the aqueous extract of Gelidiella acerosa showed reddish-brown colour, which suggested the formation of Ag- NPs in solution. The colour arises due to excitation of surface plasmon vibrations in the silver metal nanoparticles (15). The reduction of silver was subjected to analysis by using the UV-Vis Spectrophotometer. Absorption spectra of Ag-NPs formed in the reaction media has absorbance peak at 408 nm, broad-ening of peak indicated that the particles are polydispersed (Figure 1). The frequency and width of the surface plasmon absorption depends on the size and shape of the metal nanoparticles as well as on the dielectric constant of the metal itself and the surround-ing medium (16-18).
The biosynthesised silver nanostructure by using Gelidiella acerosa extract was further demonstrated and confirmed by the character-istic peaks observed in the XRD image at 2θ = 28.09o, marked with (220). A number of Bragg reflections corresponding to the (220) sets of lattice planes are observed which may be indexed based on the face-centred crystal structure of silver. The XRD pattern thus clearly shows that the Ag-NPs are crystalline in nature (Figure 2).
The SEM image (Figure 3A) showing the high density Ag-NPs synthesized by the Gelidiella acerosa further confirmed the de-velopment of silver nanostructures. The SEM micrographs of nanoparticle obtained in the filtrate showed that Ag-NPs are spherical shaped, well distributed without aggregation in solution. The silver nanoparticles synthe-sized by the help of Gelidiella acerosa extract were scanned using TEM from which the average mean size of the silver nanoparticles was 22 nm and seems to be spherical in morphology as shown in figure (Figure 3B).
FTIR analysis was used for the character-ization of the extract and the resulting nano-particles. Absorbance bands seen at 3441, 1658, 1535 and 1400 cm-1 were assigned to the stretching vibrations of primary and sec-ondary amines, respectively (Figure 4). The result revealed that the capping ligand of the Ag-NPs may be an aromatic compound or alkanes or amines.
The evaluation of antibiotic resistant patho-genic fungi has stimulated the search for ef-fective antifungal agent from alternative sources. Many studies have shown the antimi-crobial effects of nano-Ag (19-22). However, only limited literatures supports the effects of Ag-NPs against fungal pathogens. Further the nanoparticles synthesis by green route by using Gelidiella acerosa extract was found highly active against tested fungal species at a concentration of 50 μl of synthesized Ag nanoparticles. The results showed higher anti-fungal activity against Mucor indicus (22.3 vs. 21.3) and Trichoderma reesei (17.2 vs. 14.3), whereas moderate activity was revealed against Fusarium dimerum (13.15 vs. 13.0), Humicola insolens (12.2 vs. 12.1) when com-pared with standard antifungal agent Clotri-mazole (Figure 5).
Discussion :
The present study showed a simple, rapid and economical route to synthesize Ag-NPs from red seaweeds. The zone of inhibition clearly showed that the fungal strains tested were susceptible to silver nanoparticles. Thus the present study proved that the silver nano-particles synthesized from Gelidiella acerosa seem to be promising and effective antifungal agent against the pathogenic fungal strains.
Conclusion :
In conclusion, the bio-reduction of aqueous Ag+ ions by the aqueous extract of Gelidiella acerosa has been demonstrated. This green chemistry approach towards the synthesis of silver nanoparticles has many advantages such as ease with which the process can be scaled up, economic viability, etc. Applica-tions of such nanoparticles in medical and other applications makes this method poten-tially use for the large-scale synthesis of other inorganic nano materials. Toxicity studies of Ag-NPs on human pathogen opens a door for a new range of antibacterial and antifungal agents.
Acknowledgement :
The authors are grateful to the authorities of Karpagam University, Coimbatore, Tamil Nadu, India for providing facilities and for their encouragement. Authors also thank Dr. M. Ganesan, Scientist, CSMCRI-Marine Al-gal Research station, Mandapam camp, Tamil Nadu, India for the species identification.The authors would like to acknowledge Depart-ment of Nano Science and Technology, Bhar-athiar University for the XRD and SEM an-alysis. We extend our thanks to Dr. Anuradha Ashok, Nanotech Research Facility, PSG In-stitute of Advanced Studies Tamil Nadu, India for the TEM analysis.
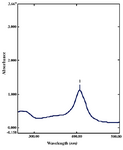
Figure 1. UV-Vis absorption spectra of silver nanoparticles synthesized from Gelidiella acerosa extract by treating 1mM silver nitrate after 48 hr
|
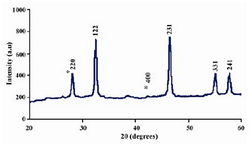
Figure 2. XRD patterns of capped silver nanoparticles synthesized by treating Gelidiella acerosa extract with
1 mM silver nitrate
|

Figure 3. SEM (A) and TEM (B) micrograph of silver nanoparticles synthesised by the reaction of 1 mM silver nitrate with Gelidiella acerosa extract
|
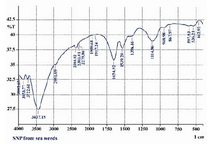
Figure 4. FTIR spectra of silver nanoparticles synthe-sized by the reduction of 1mM silver nitrate with the Gelidiella acerosa extract
|
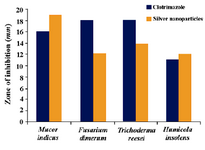
Figure 5. Antifungal activity of silver nanoparticles synthesized by the reduction of silver nitrate with the Gelidiella acerosa extract against some selected fungal pathogens
|
|