Comparative Expression Profile of Orphan Receptor Tyrosine Kinase ROR1 in Iranian Patients with Lymphoid and Myeloid Leukemias
-
Shabani, Mahdi
-
Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
-
Asgarian-Omran, Hossein
-
Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
-
Hojjat-Farsangi, Mohammad
-
Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
-
Vossough, Parvaneh
-
Clinic of Hematology, Ali-Asghar Hospital, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran
-
A.Sharifian, Ramazan
-
Clinic of Hematology and Oncology, Vali-Asr Hospital, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Toughe, Gholam Reza
-
Clinic of Hematology and Oncology, Vali-Asr Hospital, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Razavi, Seyed Mohsen
-
Clinic of Hematology and Oncology, Firozgar Hospital, Faculty of Medicine, Iran University of Medical Sciences , Tehran, Iran
-
Khoshnoodi, Jalal
-
Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
-
Jeddi-Tehrani, Mahmood
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Rabbani, Hodjattallah
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Immune and Gene Therapy Lab, Cancer Center Karolinska, Karolinska Hospital, Karolinska Institutet, Stockholm, Sweden
-
 Shokri, Fazel
Ph.D., School of Public Health, Tehran University of Medical Sciences, Tehran, 14155, Iran, Tel: +98 21 88953021 Fax: +98 21 66462267 E-mail: fshokri@sina.tums.ac.ir
Shokri, Fazel
Ph.D., School of Public Health, Tehran University of Medical Sciences, Tehran, 14155, Iran, Tel: +98 21 88953021 Fax: +98 21 66462267 E-mail: fshokri@sina.tums.ac.ir
-
Clinic of Hematology, Ali-Asghar Hospital, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
Abstract: It has recently been shown that ROR1, a member of the receptor tyrosine kinase family, is overexpressed in leukemic B cells of Chronic Lymphocytic Leukemia (CLL) and a subset of Acute Lymphoblastic Leukemia (ALL). In this comparative study the expression profile of ROR1 mRNA was investigated in Iranian patients with CLL and Acute Myelogenous Leukemia (AML) and the results were compared with those previously reported in our Iranian ALL patients. RT-PCR was performed on bone marrow and/or peripheral blood samples of 84 CLL and 12 AML patients. CLL samples were classified into immunoglobulin heavy chain variable region (IGHV) gene mutated (n=55) and unmutated (n=29) and also indolent (n=42) and progressive (n=39) subtypes. ROR1 expression was identified in 94% of our CLL patients, but none of the AML patients expressed ROR1. No significant differences were observed between different CLL subtypes for ROR1 expression. Taken together the present data and our previous results on ROR1 expression in ALL, our findings propose ROR1 as a tumor-associated antigen overexpressed in a large proportion of lymphoid (CLL and ALL), but not myeloid (AML) leukemias. Expression of ROR1 seems to be associated to lineage and differentiation stages of leukemic cells with a potential implication for immunotherapy.
Introduction :
ROR1 is a member of the family of tyrosine kinase receptors which is highly conserved among various species (1). Gene knockout studies in mice have shown the critical developmental role of ROR1 in heart and skeletal organogenesis (2). Functional ROR1 ligands have remained unknown, though secreted proteins of the wingless-type MMTV integration site (Wnt) family have recently been proposed as ligand candidates (3,4) and Wnt5a was shown to bind Ror1 (5). Ten years after identification of ROR1 (1), gene expression profiling of B cell malignancies on a genomic scale displayed ROR1 overexpression in leukemic B cells of Chronic Lymphocytic Leukemia (CLL) (6,7).
CLL represents a heterogeneous disease with a highly variable prognosis characterized by the gradual accumulation of small mature CD19+/CD5+/ CD23+ B cells (8). It has a very variable clinical course, with survival ranging from months to decades (9). The mutational status of the immunoglobulin heavy chain variable region (IGHV) genes categorizes the disease into indolent non-progressive and progressive entities which has been confirmed as an important prognostic marker in prospective clinical trials (10). Leukemic CLL cells from patients with indolent clinical course typically express mutated IGHV, whereas patients with aggressive clinical course typically express unmutated IGHV (11,12). Recently, two studies have separately demonstrated that Ror1 protein is uniformly expressed in all CLL samples independent of molecular and clinical heterogeneity or mutational status, whereas normal B cells, other normal blood cells, and normal adult tissues do not express cell surface Ror1 (11,13). Furthermore, the expression of ROR1 gene has recently been reported in both tumor tissues and Peripheral Blood Mononuclear Cells (PBMCs) of renal cancer patients (14) and non-Hodgkin lymphomas (15,16).
Our recent investigation in a group of Iranian patients with Acute Lymphoblastic Leukemia (ALL) demonstrated ROR1 overexpression in about 40% of patients (17). Little is known about the expression pattern of ROR1 in myeloid leukemias and in Iranian patients with CLL. In the present study we investigated ROR1 expression in Iranian patients with CLL and Acute Myelogenous Leukemia (AML) and compared the results with our previous findings in Iranian patients with ALL.
Materials and Methods :
Patients and controls
Heparinized Bone Marrow (BM) and/or Peripheral Blood (PB) samples were obtained from 84 CLL (only PB) and 12 AML (7 paired BM and PB, 4 BM, and one PB) Iranian patients attending the Hematology and Oncology Clinics of the Vali-Asr hospital affiliated to Tehran University of Medical Sciences and the Oncology Clinics of Firozgar and Ali-Asghar hospitals, affiliated to Iran University of Medical Sciences. A consent letter was taken from all patients or their parents and the study was approved by the Ethical Committee of Tehran University of Medical Sciences. Heparinized PB samples collected from 33 normal healthy donors (13 children with a mean age of 7.1 years and 20 adults with a mean age of 42.7 years) served as controls to determine baseline ROR1 expression and cut-off value.
Nucleotide sequence analysis of the IGHV genes of the leukemic cells has allowed classification of the CLL patients into mutated (n=55) and unmutated (n=29) groups, based on the presence of more than 2% somatic mutation compared to the germline sequence (18). The patients were clinically classified into indolent (n=42) and progressive (n=39) subtypes (19). Diagnosis of AML was based on cytomorphological findings (FAB criteria) and immunophenotypic characteristics of BM leukemic cells (20). Major demographic features of CLL and AML patients have been published (19, 20).
ROR1 PCR
PBMCs of all subjects were isolated by density-gradient centrifugation using Histopaque (Sigma, St. Louis, MO). RNA extraction and cDNA synthesis were performed as previously described (21). PCR amplification was performed using ROR1-specific primers: 5'-CTG CTG CCC AAG AAA CAG AG-3' as sense and 5'-CAT AGT GAA GGA AGC TGT GAT CT-3' as antisense (Gen Bank accession No. NM 005012) and β-actin-specific primers: 5'-ATG GCC ACG GCT GCT TCC AGC-3' as sense and 5'-CAG GAG GAG CAA TGA TCT TGA T-3' as antisense (Gen Bank accession No. NM_001101.2). Briefly, 25 µl of PCR mixture were prepared using 2.5 µl of 10× PCR buffer, 1 µl (for ROR1) and 3 µl (for β-actin) of 25 mM MgCl2, 1.5 µl dNTPs (10 mM), 0.5 µl of each primer (10 pmol/µl), 0.1 µl of Taq-DNA polymerase (5 U/µl; CinnaGen, Iran), and 3 µl (for ROR1) and 1 µl (for β-actin) of cDNA. PCR was followed by 37 cycles for ROR1 and 26 cycles for β-actin. Each cycle of ROR1 amplification consisted of 92 °C for 30 s, 62 °C for 30 s, 72 °C for 1 min and finally 72 °C for 10 min. Each cycle of β-actin amplification consisted of 92 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s and finally 72 °C for 10 min. The amplicon sizes of ROR1 and β-actin PCR products were 545 and 321 bp, respectively. PCR products were finally visualized by running agarose gel (1.5%) electrophoresis containing ethidium bromide.
Both ROR1 and β-actin products of each sample were run simultaneously in a single lane to minimize between-run variations and enhance precision of the assay. After electrophoresis, images were taken using a gel documentation system (UVP, LMS- 20E, USA). ROR1 and β-actin band densities were determined by Labworks 4.0 software (UVP, USA), and the ratio of the two bands was calculated for each sample and multiplied by 100.
Immunophenotyping of leukemic cells
Mononuclear cells from BM or PB of CLL and AML patients were immunophenotyped by flow cytometry using FITC- or PE-conjugated monoclonal antibodies (mAbs), as previously described (19,20). Staining of at least 20% of the leukemic cells after subtraction of background staining with isotype matched conjugated mAbs of irrelevant specificity was considered positive.
Statistical analysis
Analyses were conducted using the SPSS statistical package (SPSS, Chicago, IL). Statistical analyses of the results were performed using Chi-Square, Fisher’s exact tests, and Mann–Whitney U tests as appropriate. P-values of less than 0.05 were considered significant.
Results :
Relative expression of ROR1 mRNA levels in patients and normal subjects was determined by calculation of the ratio of ROR1 PCR product band density to that of β-actin. The level of expression of ROR1 mRNA was determined in all samples by visualization of the corresponding PCR product following electrophoresis. Representative RT-PCR results obtained for a number of patients and normal samples are illustrated in Figure 1.
Baseline level of ROR1 expression was assigned as mean + 1SD of ROR1/ β-actin ratio of 33 normal subjects (17). Accordingly, 4 of 33 normal samples were found to be positive. Of the 84 CLL samples 79 (94%) were positive (Figure 2). However, no significant differences were observed between indolent (n=42) and progressive (n=39) subtypes. Similarly, no substantial differences were observed between IGHV mutated (n=55) and unmutated (n=29) groups of patients (Figure 3). Comparison of the expression levels of ROR1 between CLL and normal subjects demonstrated significantly higher expression levels in CLL patients (p < 0.0001) (Figure 2). Contrary to CLL, none of the AML patients was found to express ROR1 mRNA (Figures 1 and 2). There is no significant difference of ROR1 expression between BM and PB samples of our AML patients (data not presented).
Discussion :
Lymphoid and myeloid malignancies are a diverse group of neoplasms derived from clonal expansion of lymphocytes and monocytes arrested at different stages of differentiation (22). Exploration of the immunophenotypes of leukemic cells in relation to the normal hemopoietic differentiation reveals profiles that closely mimic those of corresponding normal cells at equivalent levels of maturation (23). However, the gene expression profile of the leukemic cells is not the same as that of the normal counterpart and displays many similarities and differences (7,24-27). Gene expression profile of the leukemic B cells in follicular lymphoma, hairy cell leukemia and CLL has been shown to resemble the germinal center or the memory stage of differentiation (7,24,28,29). Nevertheless, some genes such as fibromodulin (FMOD), ROR1 and some members of the WNT gene family are selectively overexpressed in the leukemic CLL B cells and are not detected in normal B cells (7,13,24,30,31).
ROR1 has recently been extensively investigated due to its potential biological functions as a member of receptor tyrosine kinase family. Although its counterparts in other species fulfill diverse recognized biological functions, its role in human still remains unknown (32). In addition, functional ROR1 ligands have remained unknown, even though Fukuda et al showed ROR1 could bind Wnt5a (5). They found that Wnt5a induced activation of NF-κB when coexpressed with ROR1 in HEK293 cells and enhanced the survival of CLL cells in vitro, an effect that could be neutralized by post-treatment with anti-ROR1 antisera (5). It has recently been demonstrated that IL-6 activated Stat3 binds to the ROR1 promoter and activates ROR1 in CLL cells (33). Interestingly, systematic knock down of all known and putative human kinases in the human cervical cancer cell line HeLa by siRNA, has introduced ROR1 as an inhibitor of apoptosis (34), suggesting its role in etiopathogenesis of the implicated tumors.
This assumption is supported by our results of ROR1 expression in 94% and 40% of Iranian CLL and ALL patients, respectively (Figure 2 and (17)). The expression level of ROR1 in our CLL patients was significantly higher than that obtained in our earlier study for the Iranian ALL patients (17) (p<0.0001). Compatible with our results Basker et al and Daneshmanesh et al have recently shown ROR1 expression both at mRNA and protein levels in all Caucasian CLL patients investigated (11,13). At protein level, Ror1 expression is selectively expressed on the surface of B-CLL cells, whereas normal B cells, other normal blood cells and normal adult tissues do not express cell surface Ror1 (5,11,13). In addition to CLL ROR1 is also expressed in non-Hodgkin's lymphomas, with high expression in mantle cell lymphoma and moderate expression in marginal zone lymphoma (15,16), but not in major adult tissues apart from low levels in adipose tissue and at an early stage of B-cell development (15).
Interestingly, ROR1 mRNA was completely negative in all our AML patients (12/12) (Figure 2). There is only one detailed report on ROR1 expression in AML, which is in line with our results. Muller-Tidow et al have analyzed expression of all human receptor tyrosine kinases (n=56) in malignant tumors of different origins and normal control samples by quantitative real-time RT-PCR. They showed that the AML samples expressed only 20 different receptor tyrosine kinases, but surprisingly none of their 85 AML patients expressed ROR1, with the exception of only one sample with weak expression (35). No published data is available regarding ROR1 expression at protein level in AML. It is important to study more cases of AML as well as other myeloid leukemias, such as chronic myeloid leukemia and promyelocytic leukemia from different ethnic origins to prove its lack of association to the myeloid lineage.
Conclusion :
In conclusion the present study provides evidence suggesting that expression of ROR1 might be associated to lineage and differentiation stages of leukemic cells with a potential implication for immunotherapy.
Acknowledgement :
We thank Mahin Kordmahin, Tahereh Shahrestani, and Khadijeh Esmailzadeh for their invaluable technical assistance. The authors report no conflicts of interest. This study was supported by grants from Tehran University of Medical Sciences and the Ministry of Health and Medical Education of Iran.
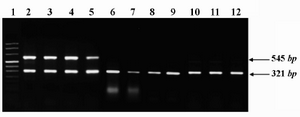
Figure 1. Representative expression profile of ROR1 mRNA in peripheral blood (PB) or bone marrow (BM) samples of a number of patients and healthy subjects. PCR amplicons of ROR1 (545 bp) and β-actin (321 bp) genes were electrophoresed on 1.5% ethidium bromide- stained gel and photographed. Lane 1: size marker; Lanes 2-5: CLL (PB); Lanes 6-9: AML (6 and 8: PB and 7 and 9: BM); Lanes 10-12: healthy subjects (PB)
|
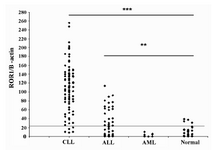
Figure 2. Relative expression levels of ROR1 mRNA in CLL, ALL and AML patients and normal subjects. The horizontal line denotes the cutoff value obtained from the normal samples; the data of ALL patients has been taken from ref 17. Relative expression of ROR1 mRNA levels in samples was determined by calculation of the ratio of ROR1 PCR product band density to that of β-actin. Baseline level of ROR1 expression was assigned as mean + 1SD of ROR1/ β-actin ratio of normal subjects.
*** : p-values of less than 0.0001
** : p-values of less than 0.005
|
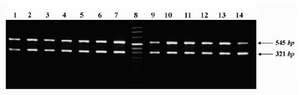
Figure 3. Representative expression profile of ROR1 in different subgroups of CLL patients. PCR amplicons of ROR1 (545 bp) and β-actin (321 bp) genes were electro-phoresed on 1.5% ethidium bromide-stained agarose gel and photographed. Lanes 1-4: patients with indolent disease; Lanes 5-7: patients with progressive disease; lane 8: size marker; Lanes 9-11: patients with mutated leukemic cells; Lanes 12-14: patients with unmutated leukemic cells
|
|