Higher Improvement of Cardiac Function Following Myocardial Infarction using Menstrual Blood Stromal/Stem Cells (MenSCs) Suspended in Conditioned Medium versus Conditioned Medium Alone in Rat Model
-
Manshori, Mahmood
-
Faculty of Veterinary Medicine, Shahrekord University,, Shahrekord, Iran
-
Nanobiotechnology Research Center, Avicenna Research Institute, ACECR,, Tehran, Iran
-
Kazemnejad, Somaieh
-
Nanobiotechnology Research Center, Avicenna Research Institute, ACECR,, Tehran, Iran
-
Naderi, Nasim
-
Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences,, Tehran, Iran
-
Shirazi, Abolfazl
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, , Tehran, Iran
-
Arabian, Maedeh
-
Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences,, Tehran, Iran
-
Darzi, Maryam
-
Nanobiotechnology Research Center, Avicenna Research Institute, ACECR,, Tehran, Iran
-
Montazeri, Samaneh
-
Nanobiotechnology Research Center, Avicenna Research Institute, ACECR,, Tehran, Iran
-
Aboutaleb, Nahid
-
Physiology Research Center, Iran University of Medical Science, Tehran, Iran
-
Department of Physiology, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran
-
 Golshahi , Hannaneh
Nanobiotechnology Research Center, Avicenna Research Institute, ACECR,Tehran, Iran, Tel: +98 21 22432020; Fax: +98 21 22432021; E-mail: h.golshahi@avicenna.ac.ir
Golshahi , Hannaneh
Nanobiotechnology Research Center, Avicenna Research Institute, ACECR,Tehran, Iran, Tel: +98 21 22432020; Fax: +98 21 22432021; E-mail: h.golshahi@avicenna.ac.ir
Abstract: Background: To evaluate the efficiency of Menstrual blood Stromal/Stem Cells (MenSCs) administration in Myocardial Infarction (MI), the effects of MenSCs and their derived conditioned Medium (CM) on cardiac function in MI rat model was assessed.
Methods: Animals were divided into four groups including sham group, MI group, MenSCs derived CM group (CM group), and MenSCs suspended in CM (MenSCs+ CM) group. The injection of different groups was carried out 30 min after ligation of left anterior descending coronary artery into the infarct border zone.
Results: The results showed a significant reduction in scar size after injection of MenSCs+CM compared to MI group. Ejection fraction and fractional shortening of MenSCs+CM group were higher than CM and MI group at day 28. Administration of MenSCs+CM led to much more survival of cardiomyocytes, and prevention of metaplastic development. Moreover, human mitochondrial transfer from MenSCs to cardiomyocytes was seen in group treated by MenSCs+CM. Indeed, MenSCs+CM treatment evoked nuclear factor-κB (NF-κB) down-regulation more than other treatments.
Conclusion: MenSCs+CM treatment could significantly ameliorate cardiac function by different mechanisms including inhibition of cartilaginous metaplasia, inhibition of NF-κB and mitochondrial transfer.
Introduction :
Myocardial Infarction (MI) is considered as one of the most important causes of fatality or hospitalization worldwide 1. In the MI process, a perplex cascade of biochemical, morphological, and molecular alterations both in infarcted and non-infarcted myocardial area happens. Indeed, necrotic cardiomyocytes are infiltrated by inflammatory cells following by granulation tissue formation, replacement of injured cardiomyocytes by the fibrotic scar tissue and remodeling of the remaining ventricle, which eventually lead to heart failure 2.
Metaplasia is known as an adaptive, benign, and reversible response to an altered environment that one type of fully differentiated and mature tissue is transformed to another type of committed tissue 3. Cartilaginous and/or osseous metaplasia are frequently found in soft tissues, however in some species such as rat and human, the cartilaginous tissue in the heart) cartilagocordis), is considered as a pathologic event 4,5. Interestingly, there are compelling evidence implying that the cartilaginous metaplasia in the heart can be the consequence of MI 6. It is supposed that hypoxia and inflammation subsequent to MI, along with mechanical stress can lead to a metaplastic change in this tissue 7,8. Nonetheless, it has been indicated that either fibroblasts or non-differentiated mesenchymal cells are capable of being transformed into chondroblasts and produce cartilaginous matrix 9.
Recently, it has been suggested that Endothelial-Mesenchymal Transition (EndMT) may induce cartilaginous and osseous formation. Moreover, inflammatory signals and/or other changes in microenvironments mediate the differentiation of endothelial-derived mesenchymal stem cells into chondrocytes and osteoblasts 10. This phenomenon can result in the reduction of the cardiac contractility, wall motion abnormalities, arrhythmia, and myocardial dysfunction 11-13. Notably, some researchers attributed cartilaginous and osseous formation as a consequence of stem cell transplantation particularly using Bone Marrow derived Stem Cells (BMSCs) 14. In contrast, other individuals that traced the feasibility of amniotic fluid stem cells in MI, postulated this phenomenon as an independent outcome of cell therapy 15.
Menstrual blood Stromal/Stem Cells (MenSCs) are known as a unique population of stem cells that can be easily harvested from women in reproductive age 16. Absence of any ethical issues, low immunogenicity, and risk of karyotypic abnormalities could be regarded as subsequent advantages of MenSCs 17,18. In addition, we recently demonstrated a higher potential of MenSCs in differentiation into cardiomyocytes as compared to BMSCs 19. Various studies have also shown that MenSCs can induce immunomodulatory properties by suppressing pro-inflammatory factors such as Interferon-γ (IFN-γ) and Tumor Necrosis Factor-α (TNF-α), and secreting anti-inflammatory cytokine like interleukin-10 (IL-10) and also by interacting with lymphocytes 20. There is some preliminary evidence on the effectiveness of MenSCs in restoration of the MI through reduction of cell death, fibrosis, scar size, and also promotion of cell proliferation, and neo-angiogenesis 21-23. However, it needs more investigation to prove the efficiency of MenSCs administration in MI restoration. Nevertheless, underlying mechanisms involved in regeneration of injured heart after stem cell therapy using MenSCs are unknown. Some studies have indicated that the beneficial effects of stem cells are mediated via the paracrine effect 24-26.
Indeed, MenSCs derived Conditioned Medium (CM) contains various growth factors, cytokines and chemokines such as vascular endothelial growth factor, epidermal growth factor, and fibroblast growth factor which can contribute to tissue regeneration 27. In our previous study, we analyzed the CM of MenSCs by growth factor membrane antibody array. We found that the MenSCs derived CM contained comparable amounts of growth factors such as insulin-like growth factor-1, basic fibroblast growth factor and epidermal growth factor 28. In this study, to find out the regenerative capability of MenSCs, their derived CM and the mechanisms that govern it, we have scrutinized the effectiveness of MenSCs accompanied by their conditioned medium (MenSCs+CM) compared to CM alone on cardiac function with the main focus on negative consequences following MI in the rat model.
Materials and Methods :
Ethical approval: Menstrual blood was collected from healthy female donors who had signed an informed consent forms. The animal experiment was approved by the Animal Ethical Committee of Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran (No: RHC.AC.IR.REC.1396.24). For in vivo study, all animals received human care in compliance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No. 85-23, revised 1985) (Care and Animals, 1986).
Isolation and Culturing the MenSCs: Menstrual blood was collected from five healthy volunteer’s women with mean age 25-35 years using a sterile Diva cup (Diva International Co., Lunette, Finland) on the second day of menstruation. Menstrual blood was transferred into the collection tubes containing 2.5 μg/ml fungizone (GIBCO, UK), 100 μg/ml streptomycin, 100 U/ml penicillin (Sigma-Aldrich, MO, USA) and 0.5 mM ethylenediaminetetra acetic acid (EDTA) in Phosphate Buffered Saline (PBS) without Ca2+ or Mg2+. Mononuclear cells were separated by a density gradient centrifugation using Ficoll-Paque (GE Healthcare, Stockholm, Sweden); centrifuged at 600 g for 20 min at room temperature and washed. Then, the cell pellet in the tube was suspended in Dulbecco’s Modified Eagle’s Medium/F12 (DMEM-F12) medium (GIBCO, UK) containing 10% Fetal Bovine Serum (FBS) and cultured in polystyrene 75-cm2 tissue culture flasks (Grainer, Frickenhausen, Germany). The flasks were kept in 37°C incubator with 5% CO2 and saturated humidity. After two days of incubation, removal of non-adherent cells was performed and the culture of adherent cells continued until 70% confluency. Adherent cells were detached using Trypsin/EDTA and passaged 29. All experiments were performed with stem cells at the 3-5 passages.
Characterization of cultured MenSCs by flow cytometry: As mentioned by Darzi S, et al 30, aliquots of 105 cells/100 μl were incubated separately with phycoerythrin (PE)-conjugated mouse anti human CD29 (clone 04-MAR; BD Pharmingen), CD73 (clone AD2; BD Pharmingen), CD44 (clone 515; BD Pharmingen), CD133 (clone TMP4; eBioscience), and CD105 (clone 43A3; BioLegend) for 40 min at 4°C.
To assess Octamer-binding transcription factor 4 (OCT-4) expression, the cells were washed with 0.1% saponin-permeabilized cells and treated with primary rabbit antihuman OCT-4 antibody (Abcam) for 40 min and then incubated with FITC-conjugated goat anti-rabbit Ig (Sigma-Aldrich) for 30 min. Isotype IgG was used as negative controls (clone MOPC-21; BD Pharmingen). Afterwards, all cell suspensions were washed twice with PBS-FBS, fixed in 1% formaldehyde solution, and analyzed using a flow cytometer (Partec GmbH, Munster, Germany) and appropriate isotype controls.
Animals: Adult male Wistar rats ranging from 10 to 12 weeks in age and weighing about 300 g were used in the present study. All animals had ad libitum access to a standard pellet diet and water. They were individually housed in polypropylene cages at a controlled ambient temperature of 24±2°C with 50±10% relative humidity and a 12-hr light/12-hr dark cycle.
Induction of MI: The animals were anesthetized with an intra-peritoneal injection of 75 mg/kg Ketamine and 5 mg/kg Xylazine and then endotracheal intubated and ventilated with a small-animal ventilator (model 680; Harvard Apparatus). The proximal portion of the Left Anterior descending (LAD) coronary artery was permanently ligated (about 2 mm from the tip of the left auricle) using a 6-0 prolene suture for induction of anterior wall MI. Successful performance of coronary occlusion was verified by the observation of a pale color development in the distal myocardium and dyskinesia of the anterior wall after ligation. The operation in the sham group was the same, except no LAD ligation was performed. Then the chest, muscle, and skin were closed with standard procedures. The animals received cefazolin (25 mg/kg), tramadol (20-30 mg/kg), and warm sterile saline (0.5-1 ml) and kept in a warm environment during the recovery period.
Preparation of CM and transplantation into MI models: CM was harvested in monolayer culture of 1.5×106 MenSCs under normoxic condition (O2 level: 20-21%). Then the animals were randomly divided into the following four groups (n=16):
Group 1 (sham), Group 2 (MI;120 μl PBS), Group 3 (120 μl CM), and Group 4 (1.5×106 MenSCs suspended in 120 μl CM). The injection of different groups was carried out 30 min after LAD ligation into the infarct border zone.
Evaluation of cardiac function: Echocardiography was performed 7 and 28 days after MI induction by a cardiologist blinded to the study group. Rats were anesthetized via administration of 75 mg/kg Ketamine and 5 mg/kg Xylazine. Echocardiography was performed for all groups with an echocardiography system (General Electric-Vingmed Ultrasound, Horten Norway). Transthoracic two-dimension-al guided M-mode echocardiography was performed using a 10 MHz electronic linear transducer. After 3 to 5 consecutive heart cycles, some of the parameters such as Left Ventricle Internal Diameter in diastole (LVIDd) and Left Ventricle Internal Diameter in systole (LVIDs) were obtained. Fractional Shortening (FS) and Ejection Fraction (EF) were calculated:
[(LVIDd–LVIDs)/LVIDd]×100 and (LVIDd2–LVIDs2) /LVIDd2 ×100.
Histological examination: After euthanasia at day 7 and 28 after MI induction, the hearts were isolated, washed with PBS. Specimen were harvested and fixed with 10% neutral buffered formalin. The samples were dehydrated, embedded in paraffin, sectioned at 5 μm, and stained with Hematoxylin and Eosin (H&E) and Masson’s trichrome to evaluate sequential histopathological alterations. The infarct size was expressed as the percent ratio of infarct area to the entire left ventricular area.
Immunohistochemical evaluation: The paraffin-embedded specimens were cut 4-µm thickness. Briefly, following sections were deparaffinized and rehydrated. The antigen retrieval step was performed by microwave heating base using EDTA buffer (pH=9) for all markers except NF-κB. For NF-κB citrate buffer (pH=6) was used. The endogenous peroxidase was blocked by 0.3% H2O2 and protein blocking was done by 2% bovine serum albumin mixed in normal sheep serum. After that, the slides were incubated with a primary antibody to collagen I monoclonal antibody [Col I], (ab34710, Abcam, 1:750), col II, [CP18], (Sigma-Aldrich ,1:150), NF-κB, (ab16502, Abcam, 1:500), anti-mitochondria, (MAB1273, Merk, 1:150), anti-α-Actinin (Sarcomeric) (A7732, Sigma-Aldrich, 1:100), and connexin-43, (C8093, Sigma-Aldrich, 1:100) at 4°C for overnight. The Envision detection system (Dako K5007-Denmark) was also used. Finally, the slides were counterstained with Mayer’s hematoxylin (Bahar afshan-Iran) for 2 min, dehydrated, and mounted and analyzed by a veterinary anatomic pathologist. The quantitative evaluation of NF-κB expression was performed using ImageJ software (ImageJ, NIH, Bethesda, MD, USA).
Statistical analysis: The quantitative results were calculated by SPSS, version 20.0, software (IBM Corp., Armonk, NY, http://www.ibm.com). One-way analysis of variance tests was used to compare the differences between groups. Data are presented as the mean±SD. p<0.05 denoted a statistically significant difference. Differences in three or more groups were tested by one-way ANOVA.
Results :
Immunophenotypic pattern of cultured MenSCs: Flow cytometry analysis was carried out to show the expression of the surface markers of MenSCs. Surface marker profiles of MenSCs were positive for CD105, CD29, CD44, and CD73 as MSC marker and OCT-4 as the embryonic marker. However, they failed to express CD133 in cultured cells and reflected a non-hematopoietic stem cell phenotype (Figure 1A).
Cardiac function: After administration of the MenSCs+CM and CM, the cardiac function outcome was measured 7 and 28 days post-MI induction by echocardiography to see whether transplantation of MenSCs+CM or CM can improve cardiac function and/or prevent further loss of heart function. Echocardiographic indexes at day 7 post-MI indicated the EF and FS were significantly decreased in MI group compared to sham approving induction of MI in rats following LAD ligation (p<0.001 and p<0.001, respectively). Instead, a noticeable improvement in EF percent was detected in treated groups (CM and MenSCs+CM) in comparison with the MI group (p<0.05 and p<0.05, respectively). A similar trend was obtained in regard to the percent of FS.
No significant difference was observed in the EF and FS of CM group and MenSCs+CM group at the mentioned time point (p˃0.05). At day 28, administration of MenSCs accompanied by CM resulted in greater percent of EF than MI and CM group (p<0.001 and p<0.05 respectively). Furthermore, the MenSCs infusion showed an upward trend in FS and EF compared to the CM-received group (p<0.05 and p<0.05 respectively) (Figures 1B and C).
Histologic evaluation: At day 7, histological situation of retrieved tissues from different groups was as following:
In the sham group, there were normally organized and anastomosing cardiac muscle fibers (Figures 2A and B). In MI group, many disrupted cardiac muscle fibers with the absence and/or decreased striation, sub-endocardial sparing, and degenerative changes were detected. Extensive granulation tissue existed around the center of necrosis. Mild to moderate accumulation of inflammatory cells was also detected. Interestingly, a marked decrease in ventricular wall thickness and an increase in chamber area was observed (Figures 2C and D).
In CM group, there were mild-to-moderate infiltration of inflammatory cells, large area of necrosis, and interstitial edema. A fibrovascular tissue containing new vascular structures filled the defect with variable density (Figures 2E and F).
In MenSCs+CM group, early granulation tissue formation with high cellularity and typically new capillary formation were noted. Remarkably, some scattered islets of myocytes had been preserved within the necrotic zone. The severity of polymorphonuclear cells was lower than those of the other groups. However, the amount of cellularity and infiltration of macrophages in the loose granulation tissue was higher than those of the other groups at this time point (Figures 2G and H).
After 28 days, in MI group, mature fibrovascular tissue within the damaged zone were detected. Infiltrations of neutrophils, macrophages, and lymphocytes were identified in the infarct areas particularly among muscle fibers with lower severity. Furthermore, cartilage formation and mineralization in the sub-endocardial areas were observed in some cases (Figures 2K and L). In CM group, the scar tissue was thin and mainly composed of a large number of fibroblasts and collagen fibers, with mild-to-moderate inflammatory cells and vascularity. Cartilage formation was also detected in the most cases of this group (Figures 2M and N). In MenSCs+CM group, there were more preserved islets of cardiomyocytes in the scar area in comparison with other groups. In addition, increased thickness of the infarct border zone ventricular wall and decreased areas of collagen deposition compared to other groups were detected (Figures 2O and P). MenSCs+CM treatment significantly diminished the infarct size at 4 weeks post-MI compared to no-treated group (p<0.05). Although the infarct size in CM-received group decreased partially, it indicated no significant difference with that of the MI group (p>0.05). While the decrease in infarct size of group treated by MenSCs+CM was higher compared to that of CM group, the difference was not statistically significant (p>0.05) (Figures 3A-B).
Immunohistochemical results: In IHC analysis, scarcity of α-sarcomeric actin-positive cardiomyocytes was seen in scar tissue of MI and CM-received groups, in contrast to ample presence of the mentioned cells in the MenSCs-transplanted group at both time points (Figures 4A-H). In addition, while the native myocardium strongly expressed connexin-43, lack of this marker was detected in the scar area of cases in MI group 7 and 28 days’ post-MI. Interestingly, partial expression of connexin-43 into spread islets of healthy myocytes was observed at the intercalated disks of MenSCs+CM group at both time points (Figures 4I-P).
IHC staining of the sections using anti-human mitochondrial antibody indicated a strong signal of human mitochondria 4 weeks after MI. Remarkably, transfer of human mitochondria was detected in cardiomyocytes in MenSCs transplanted animals (Figure 4Q).
Immunostaining of Col I showed scatter expression of this marker in cartilaginous deposition in groups with metaplastic change. In cell-administrated group, the expression of this marker was only observed in scar tissue (Figures 5A-H). Moreover, Col II was mainly localized at the chondrocytes and the extracellular matrix with obvious intensity in groups with cartilaginous deposit. However, no expression of Col II was detected in MenSCs+CM-received group (Figures 5I-P).
IHC analysis demonstrated that the expression of NF-κB was markedly enhanced in the MI group compared to the sham, CM, and MenSCs+CM (p<0.001, p<0.01, and p<0.001, respectively). Meanwhile, our result also showed that MenSCs+CM diminished the NF-κB activity more efficiently as compared to CM in the cardiac tissue (p<0.05) (Figures 6A-H).
Discussion :
Heart ischemic disease is one of the main causes of high morbidity and mortality worldwide 31. Although various medical and surgical procedures can partially improve patient outcomes, no current treatment is able to generate new contractile tissue and/or reverse ischemic change in the myocardium. With regard to significant progress in the field of cell therapy, it has developed as a promising therapeutic approach with high prospects 32.
In the present study, we demonstrated that MenSCs accompanied by CM possess therapeutic potential and appropriately protect myocardial tissue from subsequent injuries following MI with preservation of cardiomyocytes, prevention of fibrosis progress, and modulation of inflammatory reaction. It is also shown that the combination of MenSCs and their derived CM could inhibit NF-κB expression in MI model. Inhibition of NF-κB can lead to suppression of inflammatory factors such as IL-1, IL-6, and apoptosis 33. Moreover, suppression of NF-κB signaling reduces interstitial fibrosis, ventricular rupture, myocardial hypertrophy, and heart failure in the MI 34.
In our study, the metaplastic change and mineralization corroborated by typical expression of type II collagen occurred after MI in no-treated MI and CM group. Aljinovic reported cartilaginous and osseous metaplasia seven weeks after induction of MI in the rat model. They indicated that cartilage tissue formation in the rat’s heart was the result of oxygen diffusion from the oxygen-enriched blood inside the left ventricle and the local slowing of blood current inside the aneurysm following coronary artery ligation 35. Pillai et al introduced cardiac fibroblasts as a cause of bone tissue formation in myocardial tissue. It was shown that fibroblasts in the affected area greatly increased the expression of Ectonucleotide Pyrophosphatase Phosphodiesterase 1) ENPP1(, that is induced upon injury and regulates bone mineralization 36. The administration of MenSCs accompanied by CM inhibited the formation of cartilaginous tissue approved by no expression of col II in scar tissue. Col I expression was only observed in rare chondrocytes in the cartilaginous metaplastic areas; however, the expression of this type of collagen was only detected in the scar tissue of infarct zone in cell injected group with no incidence of metaplastic changes.
Impediment of metaplastic change in MenSCs injected group may be related to inhibition of NF-κB. This inflammatory mediator is known as a key developmental signaling mediator in chondrogenic differentiation and endochondral ossification. NF-κB is known to activate different signaling pathways involved in EndMT 37 along with Transforming Growth Factor β (TGF-β) 38. Indeed, activation of the NF-κB pathway is necessary for induction and maintenance of TGF-β-dependent EndMT 39. Therefore, it is suggested that MenSCs with inhibition of NF-κB expression, can prevent metaplastic formation. In this line, inhibitory effect of MenSCs on EndMT has been indicated which leads to reduction in the total number of cardiac fibroblasts and tissue fibrosis progression 22.
Interestingly, the presence of human mitochondria as a marker of injected MenSCs into injury site implys MenSCs mobilization and survival in the injured site even 28 days’ post-transplantation is demonstrated. The mitochondrial transmission to preserved rat cardiomyocytes is an interesting phenomenon that can lead to cell death suppression 40, preserve aerobic respiration 41, and increase ATP production 42. It is postulated that transfer of functional mitochondria from MSCs to cardiomyocytes can reprogram the differentiated cardiomyocytes to progenitor-like cells 43. Moreover, it is indicated that mitochondrial transfer from MSCs to endothelial cell rescues the injured endothelial cell by reducing apoptosis and promoting proliferation 44.
Despite the promoting effectiveness of stem cells in restoration of injured tissues through release of several immuno-modulatory factors, microvesicles, microRNAs, exosomes and mitochondrial transfer have promoting impression 45. The role of MenSCs+CM in cardiac function has been presented in schematic diagram (Figure 7). In agreement with these findings, some studies reported that application of CM alone does not have therapeutic effect in some pathological conditions like renal injury and MI 46,47. Vilahur et al indicated that combination of Adipose Tissue Stem Cells (ADSCs) with their CM enhances neoangiogenesis as compared to CM or ADSCs alone 48.
Conclusion :
As a corollary, our findings suggest that administration of MenSCs+CM into ischemic microenvironment post-MI, is able to preserve the cardiac viability, improve cardiac contractility by inhibiting metaplastic changes, reducing fibrosis, raising myocardium volume, and also keeping myocardium from further subsequent injuries mainly with donation of their healthy mitochondria, to cardiomyocytes. Indeed, the prevention of metaplastic changes could be considered as one of effective consequences of cell therapy using MenSCs that may contribute to cell/tissue injury recovery in myocardial infarction. Future investigation is needed to explore the exact mechanism of the MenSCs communication with the recipient cells and MenSCs+CM pathways involved to cardiac regeneration.
Acknowledgement :
This research is part of a DVM thesis of Mahmood Manshori and financially was partly supported by Avicenna Research Institute (Grant Number: 960107-018).
Conflict of Interest :
The authors declare that they have no conflicts of interest for this article.

Figure 1. Evaluation of MenSCs by flow cytometry and cardiac parameters by echocardiography at days 7 and 28 post-MI. A) CD markers are demonstrated (gray and white curves). B) % Fractional shortening (FS), C) % Ejection fraction (EF), * p˂0.05; ** p˂0.001.
|
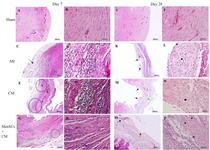
Figure 2. Histopathological evaluation of cardiac function at days 7 & 28 post MenSCs administration; A & B) Section from sham group shows normal architecture at day 7, C) In MI group, the ischemic region is mainly occupied by granulation tissue (arrow) at day 7, D) Higher magnification of a slide belonged to MI group shows a greater cellular granulation tissue (rectangle) and cardiomyonecrosis (arrow), E) In CM group, the main structure is granulation tissue (circle) at day 7, F) Higher magnification of pervious slide, notes to high cellularity within granulation tissue (rectangle), and necrotic cardiomyocytes (arrow), G) In MenSCs+ CM group, many preserved cardiomyocytes (circle) could be seen in the ischemic area at day 7, H) Higher magnification of pervious slide, note to inflammatory cells infiltration (arrow) among preserved muscle fibers (rectangle), I & J) Normal histology of section belonged to sham group at day 28, K) Note to myocardial scar tissue bulged (arrow) at day 28, L) Higher magnification of pervious image, notes to cartilaginous metaplasia (star) in the center of scar tissue, M) Note to mature granulation tissue (green arrow) and cartilaginous metaplasia (black arrow) in CM group at day 28, N) Note to cartilaginous metaplasia (star) with higher magnification in CM group O) In MenSCs+CM group, the infarct area is limited and scattered cardiomyocyte’s islets (arrow) are preserved at day 28 P) Higher magnification of pervious photomicrograph with preserved muscle fibers (arrow) (H & E, Bar=A, C, E, G, I, K, M, and O: 500 μm; B, D, F, H, J, L, N and P: 50 μm).
|
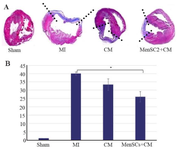
Figure 3. A) Evaluation of myocardial infarction size at day 28 post-MI, representative transversal sections of heart and Masson’s trichrome staining for infarct size measurement. Scar tissue is co-lored in blue and healthy myocardium in red. Scale bar=1.5 mm, B) Percentage of circumferential infarct size (MI size), *p˂0.05.
|
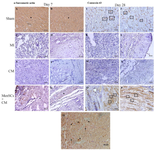
Figure 4. IHC staining of α-sarcomeric actin, connexin-43 at days 7 & 28 and tracking of transplanted MenSCs into the infarcted zone by the anti-human mitochondrial antibody. A & B) Note to noticeable expression of α-sarcomeric actin in cardiomyocytes belonged to sham group (star), C & D) No expression of α-sarcomeric actin is seen in scar tissue of MI group, E & F) No expression is detected in infarct zone of CM group, G & H) Significant expression of α-sarcomeric actin is detected in survived cardiomyocytes in MenSCs + CM group (star), I & J) Sham group, connexin-43 expression at the intercalated disks is evident (rectangle), K & L) MI group; there is no expression in scar tissue, M & N) In CM group, no expression is detectable, O & P) Notice connexin-43 expression at the intercalated disks in preserved cardiomyocytes in MenSCs+CM group (rectangle), Q) 28 days after trans-epicardial injection of MenSCs, the transfer of human mitochondria is successfully detectable in cardiomyocytes in border zone (arrow), (IHC, Scale bar = A-Q: 50 μm).
|
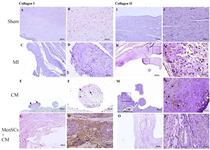
Figure 5. IHC staining of type I and II collagen in the myocardium at day 28 (A & B) No expression of Col I, (C & D) In MI group, no expression of Col I is detected in cartilaginous metaplastic site, (E) Note to limited expression of this marker by some chondrocytes in metaplastic site in CM group (arrow), (F) Higher magnification of pervious slide (arrow), (G & H) The expression of Col I in MenSCs+CM group occurs in scar tissue, (I & J) Sham group, no expression of Col II, (K) Note to expression of Col II by chondrocytes in cartilaginous metaplastic site in cases with no-treated MI (rectangle), (L) Higher magnification of pervious slide (arrow), (M) The positive expression of Col II was detectable in metaplastic region of the case that received only CM (rectangle), (N) Higher magnification of pervious slide (arrow), (O & P) There is no detectable expression of Col II in scar tissue of MenSCs+CM group (IHC, Scale bar = A, C, E, G, I, K, M and O: 200 μm; B, D, F, H, J, L, N and P: 50 μm).*p<0.05, p<0.01, and #p<0.001.
|
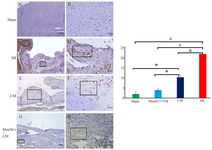
Figure 6. IHC staining of NF-κB at day 28. (A & B) No expression of NF-κB is seen in sham group, (C) Note to significant ex-pression of this marker in cardiomyocytes of border zone (circle), and cartilaginous tissue (rectangle) of scar zone in MI group (rectangle), (D) Higher magnification of pervious slide, note to expression of this marker by chondrocytes (rectangle), (E & F) Noticeable expression of NF-κB in metaplastic region (rectangle) in group that received CM, (G & H) Slight expression of NF-κB by survived cardiomyocytes in MenSCs+CM-received group (re-ctangle) (IHC, Scale bar=A, C, E, G: 200 μm; B, D, F, H: 50 μm).
|
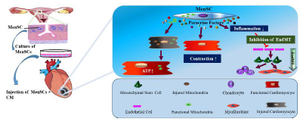
Figure 7. The role of MenSCs+CM in cardiac function.
|
|