Generation and Characterization of Mouse Hybridomas Secreting Monoclonal Antibodies Specific for Human IgG3
-
Hajighasemi, Fatemeh
-
Department of Immunology, School of Public Health, Tehran University of Medical Sciences , Tehran, Iran
-
Department of Immunology, School of Medicine, Shahed University , Tehran, Iran
-
 Shokri, Fazel
Ph.D., Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran, P.O.Box: 6446-14133, Tel: +98 21 66462268, Fax: +98 21 66462267, E-mail: fshokri@sina.tums.ac.ir
Shokri, Fazel
Ph.D., Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran, P.O.Box: 6446-14133, Tel: +98 21 66462268, Fax: +98 21 66462267, E-mail: fshokri@sina.tums.ac.ir
-
Department of Immunology, School of Public Health, Tehran University of Medical Sciences , Tehran, Iran
-
Monoclonal Antibody Research Center, Avecinna Research Center ACECR , Tehran, Iran
Abstract: Mammalians express several subclasses of the IgG molecule. In human being there are four homologous IgG subclasses, each of which is structurally unique and has different functions. Quantification of IgG subclasses is fundamental to clinical assessment and diagnosis of many diseases as such assessments depends on the availability of subclassspecific antibodies (Abs), particularly monoclonal antibodies (MAbs). In the present study, we produced and characterized two murine MAbs specific for human IgG3 molecule. These MAbs were obtained by the fusion of myeloma cells with splenocytes from Balb/c mice immunized with heavy chain of a human IgG3 myeloma protein. Fused cells were selected in hypoxanthine, aminopterine and thymidine (HAT) medium and cloned by limiting dilution assay. Ab-secreting cells were screened by enzyme-linked immunosorbent assay (ELISA) and the specificity of secreted MAbs was further analyzed, using a panel of purified myeloma proteins by ELISA and immunoblotting. Two stable hybridomas designated 1F18G7 and 1F18A11 were obtained secreting MAbs specific for Fc fragment of human IgG3. None of these MAbs showed cross-reactivity with other immunoglobulin isotypes derived from human and nine other animals, except 1F18A11 which displayed a weak cross-reactivity with only dog serum. Immunoblotting results indicate that these MAbs react with linear epitope(s) located in the heavy chain of human IgG3 molecules. The affinity constant of 1F18G7 and 1F18A11 MAbs was found to be 0.81×109 Mol –1 and 0.71×109 Mol –1, respectively, as measured by ELISA. These two MAbs with relatively high affinity can be useful tools for quantification of IgG3 subclass levels in human serum.
Introduction :
Different IgG subclasses are preferen-tially produced in response to different anti-genic stimuli depending on the nature of the antigen (Ag) and the genetic background of the species (1-3). Thus, while thymus-dependent protein Ags (TD) elicit predomi-nantly IgG1 and IgG3 subclasses, thymus-independent (TI) polysaccharide Ags tend to induce IgG2 antibody response. The profile of the IgG subclass may also be indicative of the type of the implicated disease and its severity (4-8). Analysis of the IgG subclass profiles to different antigens of a microorganism, reveals the kind of T helper associated response (9) and also is helpful for designing a potential vaccine (10).
In human there are four IgG subclasses, each of which is structurally unique with dif-ferent functions (11). This is particularly evi-dent concerning their ability to mediate com-plement activation and to bind Fc? receptors on the surface of cells involved in the immune response (12-14). While there is more than 90% sequence homology between the constant domains of ?1, ?2, ?3 and ?4 heavy chains, more marked structural differences are present in the corresponding hinge region. IgG3 mole-cules have an extended hinge region relative to other subclasses (11).
This proline-rich region confers higher flexibility and more susceptibility to proteo-lytic enzyme degradation to IgG3 molecule compared to other human IgG subclasses. Higher catalytic rate and stronger binding affinity to the Fc? receptors and the C1q com-plement component are also assumed to be associated to this unique character of IgG3 (15). A single miss-sense mutation (L368P) in the CH3 region of the human IgG3 was shown to be associated with impaired secretion of intact and functional Ig (16). Hydrophilicity and accessibility of most of the hinge amino acid residues have made this region highly immu-nogenic one to which most of the IgG3 spe-cific MAbs have so far been developed.
In the present study two MAbs specific to human IgG3 molecule were produced and characterized.
Materials and Methods :
Preparation of purified human IgG subclasses
A panel of 27 different purified human IgG myeloma proteins of known IgG sub-classes and light chain types was employed in this study. These myeloma proteins, obtained from patients with multiple myeloma, were either purified by diethyl aminoethyl (DEAE) cellulose (Whatmann, UK) chromatography or by affinity chromatography using Staphy-lococcal protein A (SPA) or Streptococcal protein G (SPG) Sepharose 4B (Pharmacia, Sweden).
The heavy chain and light chain isotypes and subclasses of myeloma were identified using isotype-specific mouse MAbs include-ing: AF6 (IgM), 8a4 (IgG), 2D7 (IgA), JA11 (IgD), C4 (?), 6el (?), JL512 (IgG1), GOM2 (IgG2), ZG4 (IgG3) and RJ4 (IgG4), kindly provided by Professor R. Jefferis (Department of Immunology, University of Birmingham, UK).
Polyclonal IgG was isolated from normal serum with SPG-Sepharose and polyclonal IgG3 was isolated, as breakthrough fraction, from polyclonal IgG by SPA-Sepharose col-umn. Fc, Fab and F (ab?) 2 fragments were prepared from several purified human mye-loma proteins of each of the IgG subclasses, by pepsin and papain digestion (17). Digested fragments were isolated by affinity chroma-tography with SPA-Sepharose column.
Animal sera
Sera from human and nine animals were obtained prepared from their clotted blood. The animals used in this study were chicken, rabbit, guinea pig, cat, dog, sheep, goat, horse and monkey. The human serum was used as the control.
???Generation and selection of hybridomas
Balb/c mice (8-12 weeks of age) were immunized with four intraperitoneal injec-tions of heavy chain from an IgG3 myeloma protein emulsified in Freund?s complete adjuvant (Sigma, U.S.A) (first injection) or incomplete adjuvant (Sigma) (other injec-tions) (50 µg every 2 weeks).
Three days after the last injection, spleen cells were fused with SP2/0 myeloma cells (NCBI C129, National Cell Bank of Iran, Pasteur Institute of Iran, Tehran), using poly-ethyleneglycol (PEG 1500) (Sigma).
Hybridomas were grown in DMEM culture medium (Sigma) containing 20% fetal calf serum (FCS) (Seromed, Germany), penicillin (100 IU/ml) and streptomycin (100µg/ml) and supplemented with hypo-xantine (1×10-4M), aminopterin (4×10-7M) and thymidine (1.6×10-5M) (HAT) (Sigma). Ten to 14 days after fusion, secreting hybrids were identified by ELISA analysis of culture supernatants as described below. Selected antibody producing cultures were cloned by limiting dilution process according to the con-ventional methods (18). Clones secreting anti-body of desired reactivity were expanded in 25 and 75 cm2 flasks (Nunc, Denmark), harvested and cryo-preserved in 40% fetal calf serum (FCS), 50% RPMI medium and 10% dimethylsulfoxide (DMSO) (Sigma).
Analysis of specificity of MAbs by indirect ELISA
Microtiter polystyrene plates (Maxisorp, Nunc, Denmark) were coated with 1-10 µg/ml of purified myeloma IgG subclasses or polyclonal IgG in PBS (0.15 M, pH=7.2). Then 0.05 ml of culture supernatant was added. Appropriate dilution of HRP-conjuga-ted sheep antimouse Ig (prepared in our lab) was subsequently added and the reaction revealed with O-phenylenediamine dihydro-chloride (OPD) (Sigma) substrate. Finally, the reaction was stopped with 20% H2SO4 and the optical density (OD) measured by a multiscan ELISA reader (Organon Teknika, Boxtel, Belgium) at 492nm.
Isotype determination of MAbs by capture ELISA
Goat antimouse IgG1, IgG2a, IgG2b, IgG3, IgA and IgM (Sigma) at 1/1000 dilu-tion, were adsorbed on to the wells of a microtitre ELISA plate (Nunc). Isotype of MAbs in culture supernatants was determined according to the ELISA technique mentioned above.
Affinity constant determination by ELISA
We determined the affinity constant (Kaff) by ELISA technique as described elsewhere (19). Briefly, ELISA plates (Nunc) precoated with three different concentrations of human IgA2 ([Ag], [Ag'] and [Ag'']) were separ
Result :
Screening and selection of specific hybridomas
Culture supernatants from growing hybridomas were screened by ELISA using a panel of four IgG myelomas with different subclasses, including the immunogen (heavy chain of IgG3). Representative results ob-tained for a number of hybridomas with different specificity profiles, including the parental 1F18G7 and 1F18A11 hybridomas are illustrated in Table 1.
Characterization of MAbs
Following cloning and subcloning, cul-ture supernatant from the selected 1F18G7 and 1F18A11 hybridomas was further charac-terized. Electrophoresis of ascitic fluids revealed a sharp monoclonal band in the ?-globulin region (Figure 1), belonging to IgG1 isotype (Table 2). Specificity of the MAbs was determined, using a panel of purified myeloma proteins, including IgG1 (n=9), IgG2 (n=4), IgG3 (n=7) and IgG4 (n=7) sub-classes. Our results demonstrated that both MAbs were specific for isotypic epitope (s) restricted to IgG3 subclass (Figure 2). The MAbs reacted only with Fc, but not Fab frag-ments of the IgG3 molecule (Figure 3). Im-munoblotting studies demonstrated that both of our MAbs recognize sequential epitopes (Figures 4A and 4B) located on human IgG3 heavy chain.
Cross-reactivity studies employing whole sera from a range of animal species indicate that MAb 1F18A8 shows a weak cross-reactivity with only dog serum (Table 3).
Determination of affinity of MAbs
The ascitic fluids of our MAbs were purified by affinity chromatography using Streptococcal protein G column and the affinity constant (Kaff) was determined by ELISA. Triple serial concentrations of the antigen (IgG3) and MAbs were selected to construct the corresponding curves and extrapolate the Kaff values using the formula given in materials and methods. Represen-tative curves obtained for the MAbs are illustrated in Figure 5 and the calculated average Kaff values are presented in Table 4.
Discussion :
In the present study we produced and characterized two murine MAbs specific for human IgG3 subclass. These MAbs were se-lected among a large collection of MAbs re-cognizing different epitopes located on human IgG subclasses. Several MAbs specific to human IgG3 subclass have been produced by other investigators (22-25).
These MAbs were shown to be specific for isotypic epitopes of IgG3 subclass, includ-ing: B12A8 specific for IgG3 (25); HP6047 (22,26), HP6050 (22,23,26), HP6010, MYSJ33 (22,23), BRL1211 (22), HP6048 and HP6066 (23), with specificity for hinge region; HP6003, HP6004 (22,23), HP6005 (23) and HP60193 (24) with speci-ficity for Fc region and HP6201 (24) specific for F(ab')2 fragments. All the above anti IgG3 MAbs, except MYSJ33 and HP6076 belonged to IgG1 subclass (like our MAbs). MYSJ33 and HP6076 MAbs belonged to IgM and IgG2b isotypes, respectively. The specificity of these MAbs was evaluated with a wide range of assay protocols (23, 24). A panel of WHO specificity reference reagents (SRR) for IgG subclasses was estab-lished (27). In the above studies purified IgG3 paraproteins (22-24) or IgG Fc region obtained from human plasma (25), were used for immu-nization of Balb/C mice while we used a heavy chain of an IgG3 myeloma for immunization. Thus our IgG3 specific MAbs can be seen useful tools for diagnosis of ?3-heavy chain diseases as well. In addition we also deter-mined the specificity of our MAbs by im-munoblotting under reduced and non-reduced conditions and determined the nature of their epitopes. The most reliable IgG3 specific MAbs produced by other investigators are HP6048, HP6050 and HP6066 which recog-nize epitopes localized in the hinge region. This specificity may preclude or complicate their application in some conditions; since it has previously been demonstrated that reduc-tion of disulfide bridges in the hinge region destroys these epitopes (23).
Our MAbs, however, recognize linear epitopes on IgG3 heavy chains (under re-duced conditions) suggesting stability of the corresponding epitope (s) and their applicabil-ity as suitable tools in different assay conditions. IgG3 subclass differs from other subclasses in hinge region and also in amino acid sequences at positions 276 and 291 in CH2 domain and positions 392, 422 and 435 in CH3 domain. In IgG1, 2 and 4 subclasses, these amino acids are Asn, Pro, Lys, Val and His, whereas in IgG3 subclass they include Lys, Ile and Arg, respectively (28). It seems that our MAbs recognize epitope(s) located in the CH2 or CH3 domain of human IgG3 subclass. The affinity constant (Kaff) of some IgG3 specific MAbs have been determined previously (24, 29). The Kaff of HP6003 and HP6004 (anti- IgG3 Fc), HP6010, HP6047 and HP6050 (anti-IgG3 hinge) has been determined by fluorescent sequential–saturation assay (29) and found to be 2.7×107, 2.5×107, 6.5×107, 3.9×107 and 5×107 Mol–1, respectively. Im-munoprecipitation studies have estimated higher avidity for HP60193, HP60194, HP6201, HP6050 and HP60195 MAbs (29). Application of different methodologies for affinity determination may explain this discrepancy in part. We measured the affinity constant of our MAbs by an ELISA-based method. The affinity constant of both of our MAbs was found in the order of 108 Mol-1 (Table 4) which is much higher than many of the previously reported IgG3-specific MAbs. One of our MAbs (1F18A8) showed a weak cross reactivity with dog serum. To the best of our knowledge this is the first report on the cross reactivity of a human IgG3 specific MAb with an animal serum. Canine IgG is composed of four subclasses which are defined as IgG1, IgG2, IgG3 and IgG4 (30, 31). Thus weak cross-reactivity of our MAb with dog serum, may suggest reactivity with dog IgG3.
Our MAbs with relatively high affinity for recognizing linear epitopes on IgG3 Fc could be used as suitable tools for quantifi-cation of IgG3 subclass in different clinical conditions and also be applied for epitope mapping o
Acknowledgement :
We are grateful to Mahmood Jeddi-Tehrani, Soheila Gharagozlou, Roya Ghods, Jalal Khoshnoodi and Azam Roohi for scien-tific consultations and preparation of the anti-gens. This study was supported in part by a grant from the Research and Technology Undersecretary of the Ministry of Health, Treatment and Medical Education of Iran.
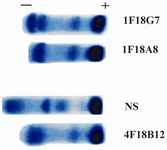
Figure 1. Acetate cellulose electrophoresis of ascitic fluids of 1 F18G7 and 1F18A8 MAbs. NS=Normal serum; 4F18B12= Anti nG1m (a) isoallotype MAb employed as a control
|
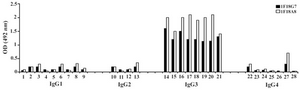
Figure 2. Reactivity of IgG3 specific MAbs with IgG subclasses Lanes 1 to 28 represent: MM1, MM11, MM24, MM46, MM50, MM53, MM63, MM65, MM13, MM12, G2 (M), MM62, Camp, MM98, Gale, Hay, Pol, Goe, Ren, Mcw, IgG3 polyclonal, MM147, Rea, Jan, Will, Cart, J. Wil, Ze
|
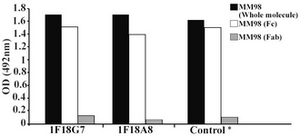
Figure 3. Reactivity of IgG3 specific MAbs with enzymatic fragments of MM98 (IgG3). Control represents a mouse MAb (HP6050) with anti-IgG3 specificity
|
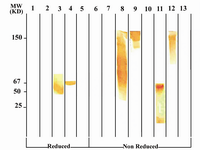
Figure 4A. Immunoblot analysis of 1F18G7 MAb reactivity with IgG subclasses. Lanes 1 to 5 represent reduced forms of MM1 (IgG1), MM12 (IgG2), Hay-ward (Heavy chain of IgG3), MM98 (IgG3) and MM147 (IgG4), respectively. Lanes 6 to 13 represent non reduced forms of MM1, MM12, Hay-ward, MM98 (whole molecule), MM98 (Fab), MM98 (Fc), IgG3 polyclonal and MM147, respectively.
|
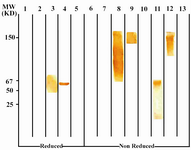
Figure 4B. Immunoblot analysis of 1F18A8 reactivity with IgG subclasses. See footnote to figure 4A
|
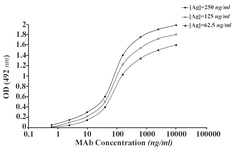
Figure 5A. Representative binding curves employed for extrapolation of affinity constant of 1F18G7 MAb.
|
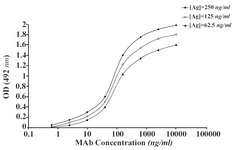
Figure 5B. Representative binding curves employed for extrapolation of affinity constant of 1F18A8 MAb.
|
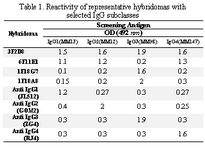
Tabel 1
|
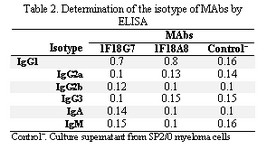
Tabel 2
|
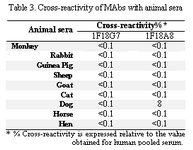
Tabel 3
|
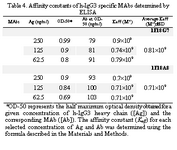
Tabel 4
|
|