Inhibitory Effects of Some Carbohydrates on Nano-Globular Aggregation of both Normal and Glycated Albumin
-
 Sattarahmady, Naghmeh
Department of Medical Physics, Faculty of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran, and Pharmaceutical Sciences Research Center, Shiraz University of Medical Sciences, Shiraz, Iran, Tel/Fax: +98 71 32349332 E-mail: nsattar@sums.ac.ir, sattarahmady@yahoo.com
Sattarahmady, Naghmeh
Department of Medical Physics, Faculty of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran, and Pharmaceutical Sciences Research Center, Shiraz University of Medical Sciences, Shiraz, Iran, Tel/Fax: +98 71 32349332 E-mail: nsattar@sums.ac.ir, sattarahmady@yahoo.com
-
Department of Medical Physics, Faculty of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
-
Nanobiology and Nanomedicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
-
Pharmaceutical Sciences Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
-
Sharifi, Esmaeil
-
Institute of Biochemistry and Biophysics, University of Tehran, Tehran, Iran
-
Heli, Hossein
-
Nanobiology and Nanomedicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Abstract: Background: Protein aggregation is one of the important, common and troubling problems in biotechnology, pharmaceutical industries and amyloid-related disorders.
Methods: In the present study, the inhibitory effects of some carbohydrates (alginate, β-cyclodextrin and trehalose) on the formation of nano-globular aggregates from normal (HSA) and glycated (GHSA) human serum albumin were studied; when the formation of aggregates was induced by the simultaneous heating and addition of dithiotheritol. For the investigations, the biophysical methods of UV-vis spectrophotometry, circular dichroism spectroscopy, transmission electron microscopy and tensiometry were employed.
Results: The effect of inhibitory mechanism of these inhibitors on the aggregation of HSA and GHSA was expressed and compared together.
Conclusion: The results showed that the nucleus formation step of the aggregation process of HSA and GHSA was different in the presence of alginate (compared to β-cyclodextrin and trehalose). The inhibition efficiencies of the carbohydrates on the aggregate formation of HSA and GHSA were different, arising from the differences in the hydrophobicities of HSA and GHSA, and also, the differences between HSA- and GHSA-carbohydrate interactions.
Introduction :
Protein aggregation has an important role in medical biotechnology, pharmacy and industry 1. In these areas, handling and storing proteins at high concentrations and temperatures for a long time induce aggregation 2. Also, protein aggregation may exist in vivo, such as protein expression at inclusion bodies or during the recovery of active proteins 3. During aggregation, proteins undergo stress and incur conformational changes which predispose them to adhesion 4.
In these conditions, structural instability and reversible unfolding occur; secondary, structural changes result in formation of intermolecular β-strands, and these are followed by reactions forming supramolecular aggregates 1,5. Formation of amorphous or strongly ordered aggregates (amyloid fibrils) is the reason of a wide range of disorders, such as Alzheimer’s and Parkinson’s disease. Amyloid formation also reduces the yield of productive refolding in biotechnological processes 6.
Regarding the importance of protein aggregation, there is an interest to characterize aggregation states of proteins and investigate biophysical features to control this process. During the protein aggregation, different processes such as conformational changes, intermolecular interactions, and solvent interactions are involved. Mechanisms of aggregation depend on the environment and physical and chemical parameters of the solvent such as temperature, pH, ionic strength, denaturant additives, etc.
Different processes such as sterilization, pasteurization and blanching applied in the food and pharmaceutical industries lead to induction of thermal aggregation of proteins 7. On the other hand, the glycation process as one of the modification reactions can spontaneously occur during a number of technological processes applied in industrial food processing and also in hyperglycemia condition in the human body 8. In this regard, many proteins such as albumin undergo the glycation process.
Cyclodextrines (CyDs) are employed in preparation of drug formulations and delivery systems 9. Trehalose (Tre) also exists in substantial quantities in modern food sources such as honey, baker’s yeasts and commercially grown mushrooms 10,11. Alginate (Alg) is widely used as a stabilizer, thickener and gelling agent in food and pharmaceutical applications and medical, agriculture and packaging fields 12.
Human Serum Albumin (HSA) with a high concentration in circulation has different functions such as drug carrying, and represents antioxidant, esterase and peroxidase activities 13,14. Both HSA and Glycated HSA (GHSA) have been selected as models as they influence the thermally and chemically induced aggregation of proteins 15-19. On the other hand, different strategies have also been used to stabilize and inhibit aggregation of proteins 20. In the present study, Alg, β-CyD and Tre, as a linear polysaccharide, cyclic polysaccharide, and disaccharide respectively, were selected to study their effects on the aggregation and unfolding states of HSA and GHSA induced by heating in the presence of DTT as thermo-chemical denaturing conditions.
Materials and Methods :
Materials: HSA (≥96%, free fatty acid), β-CyD, β-D(+) glucose, sodium Alg and sodium bicinchoninate (BCA) were purchased from Sigma (USA). Membrane filters (0.2 µm pore size, 25 mm in diameter) and dialysis tubings (cut off 10,000 MW) were from Whatman (UK). Tre, DTT, Tris buffer and sodium azide were from Merck (Germany). All other materials were of analytical grade. All solutions were prepared with deionized water.
In vitro glycation of HSA: GHSA was prepared by incubation of HSA (40 mg/ml) in a solution of sodium phosphate buffer (50 mM Na2HPO4/NaH2PO4, pH=7.4)+1 mM EDTA+0.1 mM sodium azide+100 mM glucose, in capped vials under sterile conditions at 37°C for 14 days. The protein concentration was determined spectrophotometrically before glycation using a Shimadzu spectrophotometer model UV-3100, with an extinction coefficient (E1%) of 5.30 at 280 nm 21. After dialysis of samples against sodium phosphate buffer at 4°C for 48 hr, the GHSA concentration was determined again by the BCA protein assay.
Aggregation assay and turbidity measurements: Thermo-chemical aggregation of HSA and GHSA was measured by a spectrophotometer (Shimadzu spectrophotometer model UV-3100) equipped with a water bath (±0.5oC). Protein samples of 1 mg/ml, HSA or GHSA+DTT 3.4 mM dissolved in Tris buffer (50 mM, pH=7.4), with or without additives (Alg, β-CyD or Tre), were prepared. Concentrations of Alg, β-CyD and Tre were 1% (W/V), 10 mM and 500 mM, respectively. The extent of aggregation was followed by measuring the "apparent" (due to the light scattering) absorbance at 360 nm over time (1 hr) at 65oC.
To determine the wavelength of turbidity (τ), the apparent absorbance of the protein samples was followed at different wavelengths of 350 to 650 nm at 65°C, and the changes in the apparent absorbance at 360 nm was selected as the optimum wavelength.
Transmission electron microscopy (TEM): For TEM, protein samples heated for 30 min at 65ºC were applied to 400-mesh specimen grids covered with carbon-coated formvar films. After 5 min, the buffer was removed by a filter paper and the samples were stained using 1% (W/V) methylcellulose and 1% (W/V) uranyl acetate in water. After washing, the samples were dehydrated in a graded series of ethanol 22. Transmission electron micrographs were recorded on a ZEISS electron microscope (EM 902 A) operating at 80 kV.
Circular dichroism spectroscopy: For Circular Dichroism (CD) spectroscopy, an Aviv spectrometer model 215 was employed. Far-UV CD spectra of 0.2 mg/ml of the protein samples in 50 mM Tris buffer were recorded in the range of 190 to 260 nm with a spectral resolution of 1 nm. The scan speed was 20 nm/min and the response time was 0.3330 s with a bandwidth of 1 nm. Quartz cells with an optical path of 0.1 cm were used and all the measurements were performed at 25ºC. Spectra were corrected by subtracting appropriate buffer signal and smoothed by noise reduction.
Surface tension measurements: Surface tensions of 0.2 mg/ml protein samples were measured at both 25 and 65ºC for 30 min with a Krüss K100 tensiometer based on the Wilhelmy plate method. The instrument was calibrated and checked by measuring the surface tension of distilled water before each experiment. The force of the liquid on the plate was monitored by the microbalance. The surface tension (γ) of a liquid is related to the force (F) on the plate according to the following equation:
γ=F/(L cos Θ) (1)
where L is the wetted length and Θ is the contact angle.
Results :
Structural stability and aggregation of HSA and GHSA were studied under conditions causing irreversible thermo-chemical denaturation. To investigate the relationship between aggregation and structural stability, Alg, β-CyD and Tre were employed as artificial chaperones or osmolytes to delay in aggregation.
The results of aggregation processes of HSA and GHSA in the presence of DTT with and without additives over time (1 hr) at 65oC are shown in figures 1A and 1B. The rate and total amount of aggregation are higher for GHSA, compared to HSA. In addition, figures 1A and 1B confirm the capacities of Alg, β-CyD and Tre to suppress and decrease the aggregation of HSA and GHSA. In the figures, three phases of a lag period (pre-nucleation phase), a nucleation phase (growth phase) and a saturation phase of aggregation exist. HSA and GHSA aggregated easily, and the aggregations were relatively inhibited by the additives. In the presence of Alg, growth phase in HSA and GHSA was initiated at an earlier time (lower lag phase), while the extent of turbidity significantly decreased and the curve reached plateau at a shorter time. β-CyD induced the longest delay in the pre-nucleation phase and suppressed the rate of growth phase in both HSA and GHSA. The effect of β-CyD on the total aggregation contents of HSA and GHSA were approximately the same as that of Alg. Also, Tre induced longer lag phase and slower growth phase in HSA and GHSA; however, the extent of total aggregation was higher than the states of the other additives (Figure 1).
To further compare the aggregation inhibitory effect of the additives, the morphology of the aggregates was evaluated by TEM images, as shown in figures 2A-E. Figures 2A and 2B show TEM images of incubated HSA and GHSA samples in the presence of DTT at 65°C for 30 min. Branched chains of nano-globular aggregates (70-250 nm diameters) were formed. Figures 2C and 2D show TEM images of GHSA sample incubated with β-CyD (Figure 2C) and Tre (Figure 2D). In the images, the patterns of aggregated structures in GHSA were changed, appearing as a uniformly distributed and amorphous precipitate. Figure 2E shows TEM image of the samples incubated in the presence of Alg. A pattern of a shell around aggregated cores was observed. Therefore, these additives influenced the nano-globular chain pattern of aggregation.
The surface tensions of the protein solutions under aggregation conditions were recorded. Figures 3A and 3B show the surface tensions of HSA and GHSA samples in the absence and presence of the additives at 25oC, and after heating for 30 min at 65oC. Based on the results, all the additives led to increases in the surface tensions of the solutions, and β-CyD represented the highest increment.
Figures 4A and 4B show far-UV CD spectra of HSA and GHSA. The spectra revealed that the amount of secondary structure of HSA after glycation at 25ºC had significantly less negative ellipticity. On the other hand, HSA and GHSA in the presence of the additives showed more negative ellipticity values at 208 and 222 nm.
Discussion :
Blood proteins in combination with saccharides are widely used in biotechnology, food and medical industries, and drug delivery systems. A combination of UV-vis spectrophotometry, TEM, surface tension measurements and CD technique were employed to study the effect of Alg, β-CyD and Tre on the thermo-chemical aggregation of HSA and GHSA. The aggregation propensity increases in glycation condition 3. Glycation with altering the interaction of drug and molecules with proteins, promotes dysfunction, aggregation and amyloid formation of proteins 1,2. Also, glycated form of proteins is observed in amyloid deposit 4. Knowledge about physicochemical behavior of native and glycated forms of protein in aggregation condition give insight about therapeutic approaches of amyloidosis diseases.
The results of turbidity measurements indicated that aggregation of HSA and GHSA in the absence of the additives followed a hyberbolic (parabolic) kinetic pattern after a lag period (~5 to 6 min), and reached the maximum after about 12 min. Aggregation of HSA and GHSA in the presence of β-CyD and Tre tended to have a sigmoidal (exponential) pattern with longer lag phase. However, in the presence of Alg, the lag period became shorter (2 min) and the curves were more hyperbolic. The lag phase is related to nucleus formation 23. The sigmoidal patterns of the aggregation curves for HSA and GHSA in the presence of β-CyD and Tre confirm their interactions with HSA and GHSA, stabilization of the protein structures, and induction of a delay in the pre-nucleation phase of aggregation due to different reasons such as fragmentation or heterogeneous nucleation 23. In addition, the aggregation rates of HSA and GHSA in the presence of β-CyD and Tre significantly decreased.
Some amino acid side chains participate in the aggregated nucleus formation and nucleation mechanisms and manifest themselves as lag phases. Interaction of β-CyD and Tre with proteins causes these amino acid residues to become sequestered. Therefore, the most frequent type of aggregation elimination is attained by reducing the nucleus formation to a minimum value. Some studies 24,25 have shown that the interaction of Tre with protein, through the hydrogen bonds and some amino acid side chains, reduced the exposure of hydrophobic core, and therefore prevented the aggregation of HSA and stabilized its structure. Also, β-CyD interacts with aromatic amino acids and polar residues of HSA and therefore stabilizes the three-dimensional structure of the protein 26. Regarding the impact of Alg on the aggregation of the proteins, it should be noted that both albumin and Alg have a net negative charge in the working pH and therefore, there is a preliminary electrostatic repulsion between them 27,28. However, it has been reported that there are attractions between oppositely charged parts in the albumin and Alg 29; also, Alg can mediate hydrophobic attractions with peptides and albumin without bearing hydrophobic functional groups 29,30. On the other hand, almost all of the hydrophobic residues in the albumin structure are buried inside through and between the protein helices. It has been reported that the preliminary albumin-Alg interaction is the chain segment binding between carboxyl groups of Alg and positively charged regions in the polypeptide chains of albumin 31,32. Overall, the main finding is that there are long-range repulsions between Alg and albumin and, at the same time, there are local attractions between them. Based on the results of turbidity measurements, Alg causes a preliminary denaturation of albumin to expose some hydrophobic patches and ionized protein segments. This brings about both electrostatic and hydrophobic interactions of Alg with albumin.
This analysis is also supported by a study on the binding of heat-denatured albumin to Alg 29. Based on the results of the aforementioned study, Alg binds to thermal-denatured albumin in two stages of fast and slow and also leads to a partial loss of α-helix content, intermolecular β-sheet aggregates and conformational changes in some extent at the tertiary structural level 29. Therefore, Alg initially causes albumin unfolding and denaturing to some extent and exposes the hydrophobic patches. This is just the preliminary conditions of aggregation. Comparison of the turbidity measurements indicates a higher rate of aggregate growing for GHSA, compared to HSA, in the presence of Alg. This is due to more exposure of hydrophobic patches in GHSA. In the presence of Alg, aggregate nucleus is formed in a shorter time, and the rate of nucleus growth is fast. In these conditions, assemblies of the aggregates occur in smaller collections. The TEM micrograph of GHSA in the presence of Alg showed core/shell structure which indicates entrapment or encapsulation of protein by an Alg shell around the protein. This can be related to the result of a balance between the attraction and repulsion forces between Alg and protein, which leads to a phase separation.
Stabilization of proteins in sugar medium has been related to the effect of sugar molecules on the surface tension of the solvent 33,34. Glycation of lysine and arginine residues (and maybe other charged and hydrophilic amino acid residues) in GHSA also causes alterations in the hydrophobicity/hydrophilicity of the protein surface, and decreases the surface tension. Alg, β-CyD and Tre increase the surface tension of HSA and GHSA solutions after heating at 65ºC, compared to the additive-free solutions of the proteins in the same condition. Therefore, the additives increased the surface tensions of the samples as a result of changes in geometrical dimension of the protein states 35,36. Previous studies have revealed relationships between the aggregation rate and amyloid formation increment and surface tension decrement of the protein solution 37,38, and also between protein solubility enhancement and surface tension increment 39.
Alterations in the hydrophobicity/hydrophilicity of GHSA due to attachment of carbohydrates lead to changes in the interaction of Tre and β-CyD with the protein. These changes in the exterior parts of the GHSA structure decrease the hydrophilicity of the protein surface, compared to the native surface of HSA. This induces a slightly larger amount of surface tension and inhibition in the growth of aggregation nuclei in the GHSA solution in the presence of β-CyD and Tre, compared to HSA. Therefore, an amorphous precipitate is formed from GHSA in the presence of β-CyD and Tre, compared to branched chains of nano-globular aggregates formed from GHSA without the additives.
Alg, β-CyD and Tre stabilized the HSA and GHSA structures, as evaluated by enhancement in the CD helicities through their interactions with HSA and GHSA. The interactions of β-CyD and Tre weakened the HSA and GHSA hydration and induced self-association within the protein chains 9,12. However, Alg interacts with albumin in another way. Thus, a much tighter secondary structure of α-helix becomes dominant in the proteins.
Conclusion :
Simultaneous thermal and chemical denaturation showed that aggregation of HSA and GHSA was affected by non-reducing carbohydrates. Also, Alg, β-CyD and Tre increased the surface tensions of HSA and GHSA solutions after heating at 65ºC, compared to the additive-free solutions of the proteins in the same condition. Moreover, β-CyD and Tre prolonged the lag phase of the aggregation process and β-CyD and Tre decreased the aggregation rate.
The net result of the effects of β-CyD and Tre was that these carbohydrates delayed the nucleation step of the aggregation. Alg, on the other hand, shortened the lag phase of the aggregation process. However, electrostatic repulsion between Alg and the proteins led to entrapment of the formed aggregate nuclei. Also, the net effect of Alg was the inhibition of further aggregation.
Acknowledgement :
We would like to thank the Research Council of Shiraz University of Medical Sciences (5120) and Tehran University.
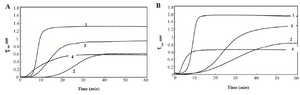
Figure 1. Turbidity at 360 nm versus time for A) HSA B) and GHSA; solutions containing DDT in the absence of additive (1) and presence of β-CyD (2), Tre (3) and Alg (4) in 50 mM Tris buffer, pH=7.4 at 65°C.
|
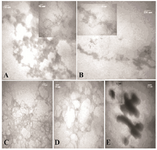
Figure 2. TEM images of A) HSA; B) GHSA; C) GHSA+β-CyD; D) GHSA+Tre; E) GHSA+Alg; after 30 min incubation in 50 mM Tris buffer, pH=7.4 at 65°C and DTT.
|
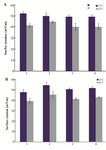
Figure 3. Surface tension of A) HSA; B) GHSA; solutions in the absence of additive (1), and presence of β-CyD (2), Tre (3) and Alg (4) in 50 mM Tris buffer, pH=7.4 at 25 and 65°C for 30 min.
|

Figure 4. Circular dichroism spectra of HSA; A) and GHSA; B) in the absence of additive 1) and presence of β-CyD (2) Tre (3) and Alg (4) in 50 mM Tris buffer, pH=7.4 at 25°C.
|
|