Neuroprotective Effects of Herbal Extract (Rosa canina, Tanacetum vulgare and Urtica dioica) on Rat Model of Sporadic Alzheimer’s Disease
-
Daneshmand, Parvaneh
-
Genetic Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran
-
Saliminejad, Kioomars
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Dehghan Shasaltaneh, Marzieh
-
Laboratory of Neuro-organic Chemistry, Institute of Biochemistry and Biophysics (IBB), University of Tehran, Tehran, Iran
-
Kamali, Koorosh
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Riazi, Gholam Hossein
-
Laboratory of Neuro-organic Chemistry, Institute of Biochemistry and Biophysics (IBB), University of Tehran, Tehran, Iran
-
Nazari, Reza
-
Laboratory of Neuro-organic Chemistry, Institute of Biochemistry and Biophysics (IBB), University of Tehran, Tehran, Iran
-
Azimzadeh, Pedram
-
Basic and Molecular Epidemiology of Gastrointestinal Disorders Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
 Khorram Khorshid, Hamid Reza
Genetic Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran, Tel/Fax: +98 21 22180138, E-mail:hrkk1@uswr.ac.ir
Khorram Khorshid, Hamid Reza
Genetic Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran, Tel/Fax: +98 21 22180138, E-mail:hrkk1@uswr.ac.ir
-
Genetic Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran
Abstract: Background: Sporadic Alzheimer’s Disease (SAD) is caused by genetic risk factors, aging and oxidative stresses. The herbal extract of Rosa canina (R. canina), Tanacetum vulgare (T. vulgare) and Urtica dioica (U. dioica) has a beneficial role in aging, as an anti-inflammatory and anti-oxidative agent. In this study, the neuroprotective effects of this herbal extract in the rat model of SAD was investigated.
Methods: The rats were divided into control, sham, model, herbal extract -treated and ethanol-treated groups. Drug interventions were started on the 21st day after modeling and each treatment group was given the drugs by intraperitoneal (I.P.) route for 21 days. The expression levels of the five important genes for pathogenesis of SAD including Syp, Psen1, Mapk3, Map2 and Tnf-α were measured by qPCR between the hippocampi of SAD model which were treated by this herbal extract and control groups. The Morris Water Maze was adapted to test spatial learning and memory ability of the rats.
Results: Treatment of the rat model of SAD with herbal extract induced a significant change in expression of Syp (p=0.001) and Psen1 (p=0.029). In Morris Water Maze, significant changes in spatial learning seen in the rat model group were improved in herbal-treated group.
Conclusion: This herbal extract could have anti-dementia properties and improve spatial learning and memory in SAD rat model.
Introduction :
Sporadic Alzheimer’s Disease (SAD) is a chronic neurodegenerative disorder which is characterized by progressive cognitive impairment, memory loss, and behavioral disturbances 1. SAD, the most controllable type of Alzheimer’s disease (AD) by drug intervention, is a multifactorial disease and affected by genetic risk factors, aging and oxidative stresses 2,3. The impairment of memory and cognition in AD patients is caused by synaptic loss, enhanced inflammatory signaling, the progressive deposition of senile plaques, neurofibrillary tangles and neurodegeneration 4-6.
Synapses are believed to be the basis of AD pathology and synaptophysin (SYP) is one of the best targets that is often measured to quantify synapses function. SYP mRNA level is reduced in the post-mortem AD brain 7-10. The amyloid-β (Aβ) peptides are implicated in the pathogenesis of AD and derived from abnormal processing of amyloid-β precursor protein (APP) by γ-secretase. Presenilin1 (PSEN1) is the catalytic subunit of γ-secretase. Neuronal inflammation and oxidative stresses activate Psen1 gene expression leading to production of Aβ plaque and synaptic dysfunction and the effect could be enhanced by hypoxia 11. Thus, abnormalities of tau and other neuron cytoskeletal proteins are correlative to the pathogenesis of AD. Among these genes, alteration in expression level of Microtubule-associated protein 2 (Map2) which has a vital role in nutrition and plasticity of the neuron was most obvious 12. On the other hand, phosphorylation of the signaling proteins also adjusts neuronal plasticity and APP processing. Among protein kinases that play such role, Mapk3 has shown significant change of expression level in AD brain and has a vital role in neurogenesis 13.
Accumulating evidences have shown that inflammatory reaction induced by Aβ results in TNF increase, oxidative stresses and memory decline. Up regulation of TNF induced by Aβ and increased levels of TNF in the brain of Alzheimer’s patient have been shown in another study 14. Brain oxidative stresses have been deeply associated with cognitive impairment and Alzheimer’s disease progression 15. Enhanced oxidative stress leads to deposition of senile plaque and synaptic loss that result in neurodegeneration. Therefore, one of the most important factors for preventing or treatment of this process is controlling of oxidative stresses. Recently, studies on novel preparation of ethanolic herbal extract from Rosa canina (R. canina), Tanacetum vulgare (T. vulgare) and Urtica dioica (U. dioica) with an immune system modulator effect, have shown positive effects on reduction of oxidative stresses and pro-inflammatory status and they seem to act as anti-aging drugs 16-20.
With respect to beneficial effects of this compound in aging, as an anti-inflammatory and anti-oxidative agent, the neuroprotective effects of the herbal extract by comparing the expression of the five important genes for pathogenesis of AD, Syp, Psen1, Mapk3, Map2 and Tnf-α between the hippocampus of SAD model treated by herbal extract and control groups were investigated. Additionally, therapeutic effects of herbal extract at behavioral, learning and memory levels were studied as well.
Materials and Methods :
Experimental animals and grouping: In this study, 40 adult male Wistar rats, weighing 250-300 g (obtained from Pasteur Institute of Iran), were housed two pair per cage with open access to food and water. They were kept in a constant environment at 22oC and 12 hr light/dark cycle. All behavioral experiments were carried out between 11 am to 4 pm.
After one week of housing, rats were randomly divided into five groups, each consisting of eight animals; the control group that received no medication and no surgery, the model group (STZ) which received bilateral Intracerebroventricular (ICV) injection of streptozocin (STZ, purchased from Sigma Chemical Co, St. Louise, USA), five days after surgery, at dose of 3 mg/kg 21, the sham-operation group (S) that received bilateral ICV injection of a-CSF, as the vehicle of STZ, the herbal extract-treated STZ group (Rose Pharmed Biomedical Inc.), which received compound 21 days after modeling 21, as I.P. at the dose of 20 mg/kg/day for three weeks 20, and the ethanol-treated STZ group (86% ethanol as I.P.) that received alcohol as the vehicle of herbal extract.
After three weeks of treatment, all groups of rats were tested for learning and memory using Morris Water Maze (MWM) test 22,23. The rats were sacrificed after MWM test and the hippocampi were immediately dissected and stored in RNA-Protector at -20oC for later RNA extraction. All experiments were conducted in accordance with the National Institute of Health Guide for the care and use of laboratory animals 24.
Surgical procedure: Based on bregma for stereotaxic surgery 21, the rats were first anesthetized with an intraperitoneal combination of ketamine (50 mg/kg) and xylazine (10 mg/kg) and then their heads were fixed in a stereotaxic apparatus. The scalp was disinfected and incised on the median sagittal of skull and the periosteom was separated. Two small holes were drilled through the skull and injection cannula was lowered into the lateral ventricles (anterior-posterior=-0.9 mm; medial-lateral=1.5 mm; and dorsal-ventral=-3.5 mm). Using a Hamilton syringe, STZ was injected 3 mg/kg and the injection was repeated on the third day with the same dose 24. The bilateral ICV injection of STZ was carried out on all groups, except for the control and sham-operated group.
Morris water maze behavioral test: Spatial learning and memory were evaluated using water maze test. The water maze consisted of a circular black tank (140 cm diameter, 60 cm height) which was filled with water (23±1oC) to a depth of 40 cm. Four equal spaces located at the periphery of the tank divided the pool into four quadrants and were used as the start position. An escape platform (11 cm diameter) was set 2 cm under the water at a constant location in the north-east quadrant of the tank. The rats were participated to a daily session of four training trials for five consecutive days 24.
The animals were subjected to a daily session of four training trials for five consecutive days, so the rat was permitted to find the platform at maximum time of 60 s and after that it was allowed to remain there for 30 s. If the rat was unable to find the platform within 60 s, it was guided to the platform by the experimenter. The amount of time spent to find the platform [Escape Latency (s)], the distance animal swam before finding it [Path Length (cm)] and the swimming speed [velocity (cm/s)] were measured.
One day after acquisition, a probe test was performed by removing the hidden platform. Rat was allowed to swim in the pool for 60 s. The times spent in the target (zone 3) and opposite (zone 2) quadrants were recorded.
To assess inability of rats to find the hidden platform, they were allowed to the pool for 60 s and four trials on sixth day. A number of behavior tests were carried out using a computer-based video tracking system 22,23.
Total RNA extraction and cDNA synthesis: Total RNA was isolated from the hippocampus tissues using RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The RNA purity and integrity were evaluated using Nano Drop ND-2000 spectrophotometer (Thermo Fisher scientific, Wilmington, USA) and gel electrophoresis.
Next, 1 μg of RNA was used to prepare cDNA with the Revert Aid First Strand cDNA Synthesis kit (Fermentas, Thermo Fisher Scientific) according to the manufacturer’s protocol.
Real-time qPCR: The relative expression levels of the five genes Syp, Psen1, Mapk3, Mtap2 and Tnf-α in hippocampus of rats in each group were detected using SYBR green real-time PCR. The real- time qPCR was performed on an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA) using Takara SYBR Master Mix instructions (Shiga, Japan). Primers were designed using Genscript and Primer3 online programs. Primer sequences and amplicon sizes are shown in table 1.
The expression of all target genes were normalized with the expression of Actb as the endogenous control 25,26. Cycle threshold (Ct) values were used to calculate fold changes in gene expression using 2 –ΔΔCt method.
Statistical analysis: Data were analyzed using SPSS 11.5 (SPSS Inc, Chicago USA). The descriptive results are shown as frequency, mean and standard deviation. For training trial test by MWM, data were analyzed by one-way ANOVA and Bonferroni post hoc test for three recorded factors (escape latency, path length and the swimming speed) between groups separately by each day for five days. The trend for each test was analyzed by repeated measure analysis. The relative gene expression was compared between groups. Statistical analysis was performed by Kruskal Wallis test. The p-value less than 0.05 was considered statistically significant.
Results :
Behavioral test: The results showed that in all study groups, the swimming distance and time for finding the hidden platform decreased during five days. The most prominent decreasing happened in the control group (normal rat); however, in the STZ induced rats’ model, the average of total swimming distance and time in five days significantly increased in comparison to the control group (p=0.001). In herbal extract-treated group, the swimming distance and time had no significant difference in comparison to control group. Significant changes in spatial learning seen in the rat model group were improved in herbal-treated group. Alcohol and sham groups had no significant difference in swimming distance and time in the total days. The swimming speed was not statistically significant between groups (Figure 1).
The results of probe trial are shown in figure 2. The STZ group spent more time in opposite quadrant than in target zone but in herbal extract-treated group, each animal spent more time in the target zone than in the opposite quadrant, compared to the untreated group. And the difference between the treated and model group was significant.
Gene expressions: Syp, Psen1 genes were found to have different expression in rat’s hippocampus after three weeks treatment with herbal extract when compared to other control groups, while the expression level of Mapk3, Tnf-a, Mtap2 did not show any difference after 3 weeks of treatment. Statistically significant change, in expression level of Syp and Psen1 were observed after three weeks of treatment with herbal extract, including a significant (p=0.001) twelve-fold increase in the expression level of Syp by herbal extract treatment (Figure 3). Meanwhile, the expression level of Psen1 significantly decreased (p=0.029) one-half-fold (Figure 4). The decrease in expression of Tnf-α was most obvious but was not statistically significant. There was no significant difference of expression in the five genes between vehicle-treated, control and modeling groups after 3 weeks of treatment.
Discussion :
Increasing evidence has supported an important role of gene expression in the initiation and progression of AD. Knowledge of the changes in the gene expression profile leads us to development of drug candidates that can reverse the changes in transcription and relieve the AD symptoms. In addition, change in gene expression probably occurs in the early stage of the disease and before the appearance of the pathological hallmarks. Therefore, drugs that can change the gene expression, can be potentially used for early treatment or prevention of the disease 27.
The loss of synaptic proteins, especially synaptophysin, is directly related to Alzheimer’s disease progression and cognitive decline. Evidence from mouse models showed that synaptophysin loss is most commonly seen in the hippocampus, than other brain regions and deficits in synapses are crucial to the development of the SAD 28,29. Interestingly, previous studies showed that SYP is a marker of SAD pathology even before formation of Aβ plaque 30. In this study, the influence of the herbal extract on Syp expression after treatment was demonstrated. In this investigation, a significant increase in Syp mRNA (induced with herbal extract treatment) was shown as well. According to previous studies, change in synaptophysin usually occurs before Aβ accumulation 31. Therefore, this result implicates that up-regulation of Syp by herbal extract treatment, could be triggered as an early option for treatment of AD.
In recent years, several studies have shown an increase of APP mRNA levels in AD brains, which exacerbate amyloid-β deposition 27. Psen1 as the catalytic subunit of γ-secretase has a known role in APP processing and Aβ formation. It was shown that significant decreases in Psen1 mRNA were induced with herbal extract treatment. Therefore, herbal compounds such as R. canina, T. vulgare and U. dioica, which potentially could reduce the expression of PSEN1, may decrease the Aβ generation and would be an effective and novel drug for AD therapy 27.
It has been shown that the Aβ interacts in a synergistic way with cytokines to induce neuronal damage 32. These events seem to be modulated by TNF-α which was up regulated in AD patient resulting in a sequence of events that lead to the generation of free radicals, oxidative damage and inflammation 33. A remarkable decrease in expression level of Tnf-α was observed after treatment with herbal extract. Interestingly, pharmacological inhibitors of TNF-α, like this herbal extract, would work as an immunomodulatory drug and improve learning and memory function. These protective effects are justified by reduction in the reactive oxygen species synthesis and synaptic disruption 34. Feedback loop of free radicals and Aβ finally leads to oxidation of protein and DNA, inhibition of ATP, neuronal damage and cognitive decline; however, the initial trigger is unknown since an age-dependent increase of oxidative stress has also been identified as an important factor leading to progression of Alzheimer’s disease. Therefore, antioxidant and anti-inflammatory reagents like herbal extract of R. canina, T. vulgare and U. dioica can work as an anti-aging agent and prevent AD progression 35.
The Morris Water Maze system was applied in this study, which is used for the research in learning and memory and is directly related to the functions of the hippocampus 23. The results of the present study suggest that this herbal extract may shorten the total swimming time and the total swimming distance of the SAD rats. In probe test for testing memory ability of rats, herbal extract could improve their strategy for searching the target quadrant zone and decrease the swimming in opposite zone. Therefore, the above results could indicate that the mentioned component could improve the spatial learning and memory in SAD rat model.
Previous studies have shown that this herbal extract treatment would markedly enhance learning and memory in an aged mouse model 20. The present study shows that 3 weeks of treatment of SAD in the rat model with this compound (20 mg/kg/day) induces a significant influence in expression of Syp and Psen1 and both of them are important for neuronal physiology, and pathogenesis of Alzheimer’s disease. As considered in Morris Water Maze, significant changes that occurred in the rat model group were improved in herbal extract treated group. The changes seen in gene expression level and behavioral test probably suggest that herbal extract of R. canina, T. vulgare and U. dioica has an anti-dementia property.
Acknowledgement :
We would like to thank Rose PharMed Co. (Iran) for providing the total herbal extract. The study was supported by the University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.

Figure 1. Trials performed in four continuous days. A) The mean value of the path length (swimming distance), B) the mean value of escape latency (time), and C) the mean value of swimming velocity (speed) in herbal extract, STZ, Alcohol, Sham and control groups.
|
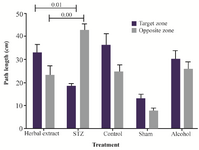
Figure 2. The mean value of total swimming distance spent in each quadrant by rat (percentage) in herbal extract, Alcohol, STZ, Sham and control.
|
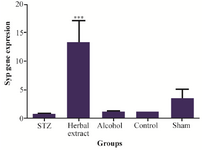
Figure 3. Comparison of synaptophysin gene expression level between the five study groups.
|
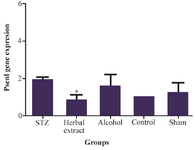
Figure 4. Comparison of Psen1 gene expression level between the five study groups.
|
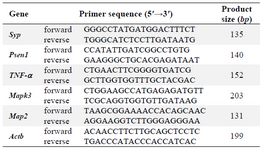
Table 1. Primer sequences and amplicon sizes
|
|