The Effect of the Duration of In Vitro Maturation (IVM) on Parthenogenetic Development of Ovine Oocytes
-
 Shirazi, Abolfazl
D.V.M., Ph.D., Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, P.O. Box: 19615-1177, Tel: +98 21 22432020, Fax: +98 21 22432021, E-mail: shiraziabbas@yahoo.com; a.shirazi@avicenna.ac.ir
Shirazi, Abolfazl
D.V.M., Ph.D., Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, P.O. Box: 19615-1177, Tel: +98 21 22432020, Fax: +98 21 22432021, E-mail: shiraziabbas@yahoo.com; a.shirazi@avicenna.ac.ir
-
Research Institute of Animal Embryo Technology, Shahrekord University , Tehran, Iran
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR , Tehran, Iran
-
Bahiraee, Amin
-
Research Institute of Animal Embryo Technology, Shahrekord University , Shahrekord, Iran
-
Ahmadi, Ebrahim
-
Research Institute of Animal Embryo Technology, Shahrekord University , Shahrekord, Iran
-
Nazari, Hassan
-
Research Institute of Animal Embryo Technology, Shahrekord University , Shahrekord, Iran
-
Heidari, Banafsheh
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR , Tehran, Iran
-
Borjian, Sara
-
Research Institute of Animal Embryo Technology, Shahrekord University , Shahrekord, Iran
Abstract: The aim of this study was to compare the effect of time of parthenogenetic activation (22 hr versus 27 hr after In Vitro Maturation-IVM) on in vitro development of ovine oocytes using either single (Ionomycin 5 ?M for 5 min or Ethanol 7% for 7 min) or combined (ionomycin and ethanol with 6-DMAP 2 mM for 3 hr) activation treatments. The abattoir-derived in vitro matured activated oocytes were cultured in modified synthetic oviductal fluid and assessed for the cleavage, blastocyst, and hatching rates. The single-activated oocytes had a reduction in cleavage, blastocyst and hatching rates compared to the combined-activated oocytes (except for the cleavage at 27 hr). In single-treated groups the rates of cleavage and blastocyst were increased as the maturation time was extended from 22 hr to 27 hr. The numbers of total cells and Inner Cell Mass (ICM), though insignificant, were greater in combined-treated groups compared to the single treatment. The number of ICM in Eth+6-DMAP group activated at 27 hr was lower than 22 hr. Nonetheless, irrespective of the activation protocol, development to the blastocyst stage, the numbers of total cell, ICM, and cell allocation (ICM/total cells) were significantly lower in parthenogenetic than fertilized embryos. In conclusion, though the cleavage and blastocyst rates in single-treated groups were positively influenced by the extension of duration of IVM (27 hr), there was a trend of decreased numbers of total cells and ICM in slightly aged oocytes. Moreover, developmental potential of ovine parthenotes, especially in young oocytes, was improved by the addition of 6-DMAP to the activation regimen.
Introduction :
Oocyte activation is one of the essential elements that determine the success of nuclear transfer and the subsequent development of cloned embryos. Failure to activate oocytes efficiently, in a simple manner constitutes one of the limiting steps for the success of cloning by nuclear transfer. Oocyte activation allows synchronization of cell cycle phase between the cytoplasm of the oocyte and the transferred nucleus, promoting nuclear reprogram-ming and maintenance of normal ploidy (1). The study of parthenogenetic activation also permits a greater understanding of the mech-anisms of spontaneous activation, preventing it in in vitro fertilization systems, and allows investigating the comparative roles of paternal and maternal genomes in controlling early embryo development (2).
Materials and Methods :
Except where otherwise indicated, all chemicals were obtained from the Sigma (St. Louis, MO, USA).
Oocyte collection and in vitro maturation
Prepubertal and adult ovine ovaries were collected at a local slaughterhouse and trans-ported to the laboratory within 2 to 3 hr in normal saline at temperature between 25 and 35°C. The ovaries were washed 3 times with prewarmed (37ºC) fresh saline, and all visible follicles with a diameter of 2 to 6 mm were aspirated using gentle vacuum (30 mmHg) via a 20 gauge short beveled needle connected to a vacuum pump. The follicle content was re-leased in preincubated hepes-buffered TCM 199, supplemented with 50 IU/ml heparin.
The method for in vitro maturation and production of sheep embryo was the same as described by Thompson et al (32) with minor modification.
Briefly, the oocytes, with at least 3 layers of cumulus cells (COCs: Cumulus-Oocyte Complexes), with a uniform granulated cyto-plasm and homogenous distribution of lipid droplets in the cytoplasm, were selected for the experiments. Before culturing, the oocytes were washed in Hepes-buffered TCM199 (H-TCM199) supplemented with 5% FBS (Fetal bovine serum, Gibco 10270), and 2 mM glutamine. The Oocyte Culture Medium (OCM) consisted of bicarbonate-buffered TCM199 with 2 mM L-glutamine supple-mented with 0.02 mg/ml cysteamine, 1 IU/ml hCG, 0.05% IU/ml FSH, 1 µg/ml E2, 100 IU/ml penicillin, 100 µg/ml streptomycin, 10% FBS (Fetal bovine serum, Gibco 10270), and 0.2 mM Na-Pyruvate. The selected COCs were pooled and randomly distributed in maturation droplets (15 oocytes in 50 µl) and covered by sterile paraffin oil in a 60 mm Petri dish (Falcon 3004; Becton & Dickinson, Franklin Lakes, NJ) and were then incubated for 22 and 27 hrs at 39 ºC under an atmos-phere of 5%CO2 and 100% humidity.
Preparation of sperm and In Vitro Fertilization (IVF)
After IVM, the oocytes were washed four times in H-SOF (HEPES- synthetic oviductal fluid) and once in fertilization medium and were then transferred into the fertilization droplets. Fresh semen was collected from a Lori-Bakhtiari ram of proven fertility. For swim up, 80-100 µl of semen was kept under 1 ml of BSA-HSOF in 15 ml conical tube at 39 ºC for up to 45 min. After swim up, the 700-800 µl of medium was gently taken off the top of the suspension and were then added into 15 m1 conical tube containing 3 ml of BSA-HSOF, centrifuged twice at 200×g for
3 min and the final pellet was re-suspended with BSA- HSOF. The oocytes were insemin-ated with 1.0×106 normal, motile spermato-zoa/ml. The fertilization medium was SOF (as originally described by Tervit et al) (33) and enriched with 20% heat inactivated estrous sheep serum. A 5 µl aliquot of sperm suspen-sion, (1.0×106 sperm/ml), was added into the fertilization droplets (45 µl) containing 10 oocytes. Fertilization was carried out by co- incubation of sperm and oocytes for 22 hr at 39 ºC in an atmosphere of 5% CO2 in humidi-fied air. After IVF, presumptive zygotes were denuded of surrounding cumulus cells by vortexing for 2 min in H-SOF containing 0.1% hyaluronidase and were then transferred to culture drops.
Activation of oocyte
Methods for activation of oocytes were modified from Susko-Parrish et al (34). After IVM (22 and 27 hrs), cumulus cells were removed by incubation in H-SOF containing 0.1% hyaluronidase at 39 °C for 2 min fol-lowed by vortexing for 3 min. Denuded oocytes were pooled and randomly allocated into single or combined treatment groups. In single treatment groups the oocytes were treated with either Ionomycin (5 µM for
5 min) or ethanol (7% for 7 min). After 5 min exposure to Ionomycin, the oocytes were then rinsed in H-SOF containing 30 mg/ml BSA to stop activation. All of the chemicals for oocyte activation were dissolved in H-SOF medium supplemented with 1 mg/ml Bovine Serum Albumin (BSA), except ethanol which was supplemented with 0.1 mg/ml PVP. In combined treatment, after oocyte activation with the same concentrations of<
Result :
Effect of oocyte age on development of parthe-notes
As shown in Table 1, two maturation times were considered to compare the effect of four activation regimens on parthenogenesis of ovine oocytes matured in vitro. The cleavage rates of artificially activated oocytes after 22 hr of culture, in groups receiving either Io or Eth was lower than groups receiving Io+6-DMAP and Eth+6-DMAP (p<0.001). How ever, the difference between Eth and Eth+6-DMAPgroups was not significant (p>0.05). The cleavage rate in the combined treatment groups (Io+6-DMAP and Eth+6-DMAP) was comparable with the IVF group. There was not significant difference between the IVF and the artificially activated oocytes after 27 hr of culture in terms of cleavage rate (p>0.05). The cleavage rates were significant-ly increased in groups Io and Eth when the maturation time was extended from 22 to
27 hrs (p<0.05).
The blastocyst rates of artificially activated oocytes after 22 hr of culture in groups receiving either Io or Eth was lower than groups receiving Io+6-DMAPand Eth+6-DMAP (p<0.001). The blastocyst rates in groups receiving Io+6-DMAP and Eth+6-DMAP after 27 hr of culture was higher (p<0.001) than groups receiving either Io or Eth (except for Eth+6-DMAP and Io). The corresponding value in IVF group, however, was significantly higher than parthenogenet-ically activated oocytes at both 22 and 27 hrs of culture (p<0.001). The blastocyst rates were significantly increased in groups Io and Eth when the maturation time was increased from 22 to 27 hrs (p<0.05).
The hatching rates in combined treatment groups after both 22 and 27 hrs of culture were significantly higher than single treat-ment groups (p<0.001). The corresponding rate, however, was significantly higher in IVF group compared to the artificially activated oocytes after 22 hr of culture (p<0.001). The hatching rate was significantly increased in group Io+6-DMAP as the maturation time was increased to 27 hr (p<0.05).
Effect of oocyte age on cell number of partheno-genetically produced blastocysts
The cell numbers in parthenogenetically developed blastocysts were lower than (p<0.001) in vitro produced embryos for 22 and 27 hrs of oocyte maturations (Table 2, Figure 1). A comparison between blastocyst cell numbers in embryos derived from the oocytes matured in vitro for 22 and 27 hrs did not show any significant difference among experimental groups (p>0.05). The number of inner cell mass and its proportion to the total cells were significantly higher in IVF derived blastocysts than parthenogenetically de-veloped blastocysts for 22 and 27 hrs of oocyte maturation (p<0.001). In group Eth+6-DMAP, the mean number of ICM was significantly higher in oocytes activated after 22 hr of culture compared to 27 hr (p<0.05).
Discussion :
The most prominent manifestations of oocyte aging include an increased susceptibil-ity to activating stimuli (13,24), most probably through a decrease in MPF activity (12,35,36), the onset of anaphase II (36,37), and partial exo-cytosis of cortical granules (38). It is known, however, that fertilization or artificial acti-vation of aged oocytes resulted in abnormal development (39,40). In this study, we were interested in testing the effects of the matur-ation time (oocyte age) on efficiency of chem-ical activators for activating ovine oocytes matured in vitro. The synergistic effect of Ionomycin and ethanol with 6-DMAP (com-bined treatments) was also investigated.
The current results indicated that in young oocytes (maturation time; 22 hr) the cleavage rate was higher in parthenogenetically acti-vated oocytes treated with combined treat-ment compared with single treatment (Iono-mycin or ethanol alone). In slightly aged oocytes (maturation time; 27 hr) there was no such difference between activated oocytes treated by either single or combined treat-ments. On the other hand, the cleavage rate in the oocytes receiving single treatment (Iono-mycin or ethanol) was positively influenced, as the age of oocytes was increased. Similar-ly, the blastocyst formation and hatching rates were positively influenced by the synergism between Ionomycin or ethanol with 6-DMAP at both 22 and 27 hrs.
In the single treated groups, the blastocyst formation rates were significantly higher in the oocytes parthenogentically activated after
27 hr of culture compared with 22 hr. The hatching rate was significantly increased in group Ionomycin+6-DMAP in oocytes acti-vated at 27 hr of culture compared to the
22 hr. These findings was in agreement with earlier studies demonstrating the low response of young oocytes to parthenogenetic acti-vation (24,25,41) and those studies in which aged oocytes were often used as recipient oocytes for nuclear transfer (18,42).
In this context, the potential of the aged mouse (26) and rabbit (16) NT oocytes receiving embryonic nuclei to develop into blastocyst was higher than that of young oocytes. In contrast, in some species following the com-bined treatment the activation rate and the proportion of parthenogenetically activated oocytes developed to blastocyst stage in young oocytes was higher than aged counter-parts (18,28). The aged oocytes, however, had higher activation rates but lower developmen-tal potential than young oocytes (6). Similarly, a higher development has been reported in the reconstructed oocytes cloned with young recipient oocytes (18). In artificially activated bovine oocytes, no differences in the percent-ages of cleavage and blastocyst development were observed between the oocytes matured for 20 and 24 hrs (43).
One possible explanation for this discrep-ancy between the current result and other reports could be due to the narrow age gap (5 hr) between treatment groups (22 and
27 hrs). There are, probably, other contrib-uting factors such as the pattern of oocyte maturation (in vivo or in vitro) and the presence or absence of cumulus cells (44) and inter-species differences which could influ-ence the responsiveness of oocytes to artifi-cial activators. There is even report indicating that the effect of oocytes aging on activation rate is strain-dependent (45).
It is known that calcium ionophore (A23187) treatment can induce a single intra-cellular calcium rise in MII oocytes and its consequence is the activation of several cal-cium dependent proteolytic pathways, leading to the destruction of cyclin B, reduction of MPF activity, and resumption of meiosis (46). In young oocytes, A23187 treatment alone caused a slower decrease in H1 kinase activity and no evident of MAPK alteration (19). In contrast in aged oocytes, activities of both kinases decreased after A23187 treatment, similar to the response in the combined treat-ments (A23187+6-DMAP). This may explain the higher proportion of cle
Acknowledgement :
The authors would like to thank the Re-search Institute of Animal Embryo Technol-ogy for technical and financial supports, Shahrekord University, Dr. A. Kheiri for his kind assistance in statistical analysis, Shooli Research Center and Shahrekord’s slaughter-house staff for their cooperation.
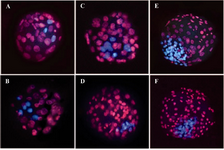
Figure 1. Epifluorescent microscopic images of bovine blastocysts derived from parthenogenetic activation and IVF. Trophectoderm and inner cell mass nuclei were labeled with propidium iodide (red) and Hoechst 33342 (blue), respectively. (A-B) Partenogenetic blastocysts derived from single treated groups (Ionomycin or ethanol). C-D) Partenogenetic blastocysts derived from combined treated groups (Ionomycin or ethanol with 6-DMAP). E-F) IVF-derived blastocysts
|
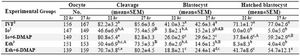
Table 1. Comparison of in vitro development of ovine oocytes activated by Ionomycin or Ethanol alone or in combination with 6-DMAPat 22 or 27 hr after in vitro maturation
a,b,c,d Means�SEM; different lowercase letters indicate statistical differences into columns (p<0.001)
A,B Means�SEM; different uppercase letters indicate statistical differences in the same embryonic development stage (p<0.05)
1In vitro fertilization; 2Ionomycin; 3Ethanol
|
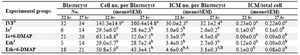
Table 2. Comparison of cell numbers in parthenogenetically developed blastocyst derived from ovine oocytes activated at 22 or 27 hr after in vitro maturation
a,b Means � SEM; different lowercase letters indicate statistical differences into columns (p<0.001)
A,B Means � SEM; different uppercase letters indicate statistical differences into rows of each subject (p<0.05).
1In vitro fertilization; 2Ionomycin; 3Ethanol
|
|