The Potential of Brittle Star Extracted Polysaccharide in Promoting Apoptosis via Intrinsic Signaling Pathway
-
 Baharara, Javad
Research Center for Animal Development Applied Biology, Mashhad Branch, Islamic Azad University, Mashhad, Iran, Tel: +98 511 8437092 E-mail: baharara78@gmail.com
Baharara, Javad
Research Center for Animal Development Applied Biology, Mashhad Branch, Islamic Azad University, Mashhad, Iran, Tel: +98 511 8437092 E-mail: baharara78@gmail.com
-
Amini, Elaheh
-
Research Center for Animal Development Applied Biology, Mashhad Branch, Islamic Azad University, Mashhad, Iran
Abstract: Background: Anti-cancer potential of marine natural products such as polysaccharides represented therapeutic potential in oncological researches. In this study, total polysaccharide from brittle star [Ophiocoma erinaceus (O. erinaceus)] was extracted and chemopreventive efficacy of Persian Gulf brittle star polysaccharide was investigated in HeLa human cervical cancer cells.
Methods: To extract polysaccharide, dried brittle stars were ground and extracted mechanically. Then, detection of polysaccharide was performed by phenol sulfuric acid, Ultra Violet (UV)-sulfuric acid method and FTIR. The anti proliferative activity of isolated polysaccharide was examined by MTT assay and evaluation of cell death was done through morphological cell changes; Propodium Iodide staining, fluorescence microscopy and caspase-3, -9 enzymatic measurements. To assess its underlying mechanism, expression of Bax, Bcl-2 was evaluated.
Results: The polysaccharide detection methods demonstrated isolation of crude polysaccharide from Persian Gulf brittle star. The results revealed that O. erinaceus polysaccharide suppressed the proliferation of HeLa cells in a dose and time dependent manner. Morphological observation of DAPI and Acridine Orange/Propodium Iodide staining was documented by typical characteristics of apoptotic cell death. Flow cytometry analyses exhibited the accumulation of treated cells in sub-G1 region. Additionally, polysaccharide extracted induced intrinsic apoptosis via up-regulation of caspase-3, caspase-9 and Bax along with down-regulation of Bcl-2 in HeLa cells.
Conclusion: Taken together, the apoptosis inducing effect of brittle star polysaccharide via intrinsic pathway confirmed the anti tumor potential of marine polysaccharide. Therefore, these findings proposed new insight into anti cancer properties of brittle star polysaccharide as a promising agent in cervical cancer treatment.
Introduction :
Cervical carcinoma is the second most threatening health problem for women worldwide in incidence and mortality 1. Some chemopreventive polyphenol agents such as curcumin, ferulic acid and resveratrol are believed to inhibit proliferation of transformed cells by apoptosis induction, DNA fragmentation and regulation of signal transduction pathways. In spite of improvement in cancer treatment strategies, still cervical cancer is the second most common malignancy in women. However, numerous researches were conducted to discover bioactive natural products with health promoting effects and short term toxicity in cervical lesions 2.
Apoptosis or programmed cell death is a type of cell death which eradicates unwanted cells and redundant tissues to maintain homeostasis. Tumor cell apoptosis is accounted as a potent defense mechanism for pre vention of cancer by regulating tumor cell cycle disturbance and it provides an alternative strategy in cancer treatment 3. Natural products are structurally diverse compounds with anti tumor activity and low side effects that improve human health and have pivotal function to eliminate tumor cells by modulation of apoptotic signaling 4.
Polysaccharides are various types of biopolymers that play crucial roles in biological events such as signal recognition, cell to cell communication and pathogenesis prevention. For instance, glycosaminoglycans such as heparin and dermatan sulfate are paid more attention due to anticoagulant activity 5.
Tremendous evidence shows that natural polysaccharides possess therapeutic potential as anti-mutagenic, anti-bacterial, anti-coagulant and anti-cancer activity. Moreover, the biological effects of polysaccharides obtained from marine ecosystem provide novel medicinal option against traditional plant polysaccharides in drug discovery 6.
As the main part of marine, polysaccharide is obtained from bacteria and algae, therefore, the extraction and identification of applicable natural polysaccharide from marine invertebrate is an interesting topic for future researches in cell therapy 7. Furthermore, holothurian (sea cucumber) among echinodermata is considered as a natural resource of sulfated polysaccharides. For example, fucoidan is a typical sulfated polysaccharide extracted from brown seaweed and sea cucumber and it possesses biological properties such as antioxidant, hepatoprotective and hypercholesterolemic effects 8. Collagen and acidic polysaccharide are composed of chief polysaccharides in body wall of sea cucumber. Further, it has been documented that fucosylated chondroitin sulfate and fucan extracted from sea cucumber’s body wall show biological potency such as anticoagulant, anti angiogenic and anti-metastatic effects 9.
Brittle stars (ophiuroidea) are marine invertebrates belonging to echinodermata and one of their more prominent features is their capacity for arm regeneration that has received little attention in the literature for its biomedical application 10. To date, the presence of some bioactive substances such as terpenes, sulfated sterols, carotenoid sulfate, phenylpropanoid and naphthoquinone in brittle stars has been proved which may have important role in anticancer therapy 11.
Furthermore, the existence of cerebrosides and alkaloids isolated from brittle star has been reported but no reports related to extraction of polysaccharide from brittle star exist 12. Since there is little information associated with extraction and biomedicine activities of marine invertebrates polysaccharides, therefore, extraction of natural marine polysaccharides from brittle stars that could induce apoptosis is fascinating 13. Hence, the aim of this study was to extract polysaccharide from Persian Gulf brittle star Ophiocoma erinaceus (O. erinaceus) and evaluate the inhibitory effect of O. erinaceus polysaccharide on human HeLa cervical cancer and ultimately address molecular mechanism.
Materials and Methods :
HeLa cells (human cervical cancer cells) were purchased from NCBI (National Cell Bank of Iran). DMEM Medium, FBS (Fetal Bovine Serum), trypsin-EDTA and antibiotic (penicillin-streptomycin) were obtained from Gibco-USA. Specimens of the brittle star (O. erinaceus) were obtained from rocky intertidal flats of Persian Gulf waters. Methanol and ethanol were purchased from Merck (Germany). MTT (3-[4, 5- dimethyl thiazol-2-yl]-2,5-diphenyl tetrazolium bromide) and DAPI (4′,6-Diamidino-2-phenylindole dihydrochloride) were prepared from Applichem (USA). PI (Propodium Iodide) and AO (Acridine Orange) were obtained from Sigma (USA). Caspase-3 and caspase-9 calorimetric assay kits were purchased from Abcam (England). This experiment was performed at Research Center of Applied Biology of Mashhad, Islamic Azad University in 2013.
Preparation of polysaccharide: To perform extraction of polysaccharide from O. erinaceus, the samples were dried, minced and extracted (Figure 1) 14.
Phenol-sulfuric acid, sulfuric acid- UV reaction and FTIR as an analytical method: To identify polysaccharide using phenol-sulfuric acid method, carbohydrate solution was mixed with phenol and concentrated sulfuric acid to reach color and the light absorption was recorded at 490 nm. In sulfuric acid-Ultra Violet (UV) method, carbohydrate solution was mixed with concentrated sulfuric acid and UV light absorption at 315 nm was recorded using UV-spectrophotometer 15. To identify the functional groups of the active compound, FTIR (Fourier Transform Infrared Region) in the region of infrared radiation was utilized.
Cell culture and maintenance: HeLa cells were grown in DMEM medium possessing 10% FBS (Gibco, USA) supplemented with L-glutamine (Sigma, USA) and 1% antibiotic at 37ºC in a humidified, 5% CO2 incubator.
Cell proliferation assay: To assess the effects of O. erinaceus total polysaccharide on HeLa cells growth, MTT assay was performed. To conduct this assay, HeLa cells were plated at a concentration of 104 cells/well in 96-well plates overnight. Then the cells were incubated with different concentrations of total polysaccharide (100, 50, 25, 12.5, 6.25, 0 µg/ml) to obtain inhibitory concentrations. Stock solution of total polysaccharide was diluted in DMEM medium. After the desired time (24 hr and 48 hr), viability of cultured cells was determined by MTT assay and the optical absorbance of dissolved formazan was measured at 570 nm spectrophotometerically by (Epoch, USA).
Morphological observation under inverted microscope: In order to investigate the effect of crude extracted polysaccharide on cell morphology, HeLa cells were seeded in 24-well plates (10 5 cells/well) and after 24 hr adherence of cells, treatment was performed at inhibitory concentrations (50% and up to 50%) for 24 hr. The cells were examined under inverted microscope (Bio Photonic, Brazil) and photographs were taken.
Acridine orange/ propodium iodide and DAPI staining: The cultured cells were treated with inhibitory concentrations of total polysaccharide for 24 hr, then, harvested and stained with 10 µl (100 µg/ml AO and 100 µg/ml PI mixture). For DAPI staining, HeLa cells were seeded on cover slips, then incubated with inhibitory concentrations of total polysaccharide for 24 hr. Next, the cells were fixed with methanol for 5 min and exposed with 100 µg/ml DAPI for 15 min at 37ºC in the dark. Ultimately, the cells were visualized under fluorescence microscope.
Detection of apoptosis: Seeded HeLa cells were incubated with medium containing inhibitory concentrations of total polysaccharide extracted from O. erinaceus overnight. Then, cells were washed with PBS and suspended with PI solution containing 0.1% sodium citrate plus 0.1% Triton X100 at 37ºC for 30 min and then placed at 4ºC in the dark for 10 min and the apoptotic cells were evaluated using a FACScan laser flow cytometer (FACSCalibur, Becton Dickinson, USA).
Determination of the type of apoptotic pathway induced by caspase-3 and -9 assays; Colorimetric caspase-3 and -9 kits were utilized for measurement of enzymatic activity on the basis of cleavage of p-nitroaniline (pNA) from labeled substrate DEVD-pNA and p-nitroanilide (pNA) and from labeled substrate LEHD-p-NA by active enzymes. Briefly, 3×106 plated cells were incubated for 24 hr with inhibitory concentrations of brittle star extracted polysaccharide. At the next step, the treated cells were trypsinized and incubated with 300 µl lysis buffer for 10 min on ice and centrifuged at 4ºC to obtain supernatant rich from cytosolic protein content. Then, the lysates were examined for measurement of caspase-3 and caspase-9 activity according to manufacturer’s protocol. Finally, the absorbance of chromophore p-NA was determined spectrometrically at 405 nm (Epoch, USA).
Statistical analysis: The statistical significance was analyzed by SPSS software through Student’s t test. All results were repeated at least three times and expressed as mean±SEM. For all comparisons, the level of p≤0.05 was considered significant.
Results :
Identification of crude polysaccharide with colorimetric assay and FTIR: On the basis of phenol-sulfuric acid method, dehydrated carbohydrates produce detectible color as well as furfural derivative which can be used for detection of sugars. UV-sulfuric acid method can recognize sugar content without the need to separate color development. However, both methods confirmed extraction of polysaccharide from O. erinaceus. In UV-sulfuric acid reaction, the absorbance of D-GLC and O. erinaceus extracted polysaccharide at 315 nm was 1.214, 1.015, and in phenol-sulfuric acid method, it was recorded as 1.52, 1.27, respectively (Figure 2).
O. erinaceus total polysaccharide suppressed proliferation of HeLa cell: The cytotoxic effect of total isolated polysaccharide was evaluated by MTT assay. As indicated in figure 3, extracted polysaccharide inhibited HeLa cell growth in a dose and time dependent manner in 24 and 48 hr, so that, the IC50 values were calculated as 25, 12.5 µg/ml, respectively.
Morphological examination: Morphological alteration of HeLa cells after treatment with brittle star extracted polysaccharide indicated that crude polysaccharide induced the morphological characteristic of apoptosis in HeLa cells such as cell shrinkage, reduction of cell volume, plasma membrane blebbing, nuclear condensation and apoptotic bodies (Figure 4A).
Analysis of apoptosis by fluorescence microscopy : Apoptosis induction is considered as a key modality in cancer fighting. Cellular alterations such as chromatin condensation and caspase cascade have characterized apoptotic cells. In this study, the typical morphological changes were assessed by fluorescence microscopy. In AO/PI staining, red color indicates necrotic cells in which PI penetrates the disturbed membrane, orange cells indicate apoptotic cells that are features of condensed chromatin and green color distinguishes intact untreated cells. As shown in figure 4, crude polysaccharide treated cells were apoptotic dose dependent and necrotic cells had higher concentration (Figure 4B). DAPI as a fluorescence DNA binding agent was applied to assess nuclear apoptosis. Staining with DAPI revealed DNA fragmentation and nuclear pyknosis (features of apoptotic nucleus) in a dose response manner in HeLa treated cells which were compared with untreated cells with normal intact nucleus (Figure 4C).
Evaluation of apoptosis in HeLa cells treated with O. erinaceus crude polysaccharide: Apoptosis was further studied by PI flow cytometry. PI staining was examined to detect cell membrane integrity and DNA fragmentation. Sub-diploid peak in this assay resulted from endonuclease activation and DNA fragmentation which is represented in HeLa cells treated with O. erinaceus crude polysaccharide and is considered to be indicative of loss of cell membrane integrity (Figure 5).
Induction of intrinsic apoptotic pathway by activation of caspase-3 and caspase-9: To evaluate the ability of caspases in induction of apoptosis by O. erinaceus crude polysaccharide, the absorbance of the enzyme activity was measured after 24 hr treatment with IC50 value in HeLa cells. The colorimetric assay revealed significant enhancement in both caspase-3 and caspase-9 activity in treated cells in a concentration-dependent manner as compared to control which showed that O. erinaceus extracted polysaccharide induced apoptosis via mitochondrial apoptotic pathway in HeLa cells (Figure 6).
Effect of brittle star extracted polysaccharide on Bax and Bcl-2 mRNA expression in HeLa cells: The mRNA expression levels of two apoptotic-related genes, Bax and Bcl-2 in human cervical cancer HeLa cells treated with inhibitory concentrations of extracted polysaccharide were evaluated using the RT-PCR. The RT-PCR analysis indicated that pretreatment of HeLa cells with isolated polysaccharide decreased Bcl-2 mRNA expression levels in a dose dependent manner and the Bax mRNA level increased compared to the untreated cells (Figure7).
Discussion :
To date, cancer is considered as a dreadful complicated disease worldwide. Considerable variation in response to drugs identifies the death induced in cancer cells. Apoptosis postpones progression of tumors with challenging high proliferative capacity of cancer cells. Apoptosis based therapy solves complications of low efficacy and drug resistance culminating in better clinical outcomes 16. Natural products as secondary metabolites play vital role in anti-neoplastic strategies with useful chemotherapeutic agents. In cancer treatment, protective effects of natural products and unique structures provide a valid alternative in chemotherapy. Due to unknown marine ecosystem, the isolation and identification of bioactive marine natural products introduced an alternative route for treatment of cancer and other degenerative disorders with apoptosis targeted therapy 17.
Sensitivity of target cells caused the apoptosis of cancer cells or non-apoptotic cell death. In malignant cells, recruitment of apoptosis has been accounted for limiting cancer progression that encourages the researchers to discover apoptosis inducing compounds 18.
Caspases are cysteine proteases that play crucial role in apoptotic cell death. Extrinsic or death receptor and mitochondrial-mediated intrinsic apoptotic pathways comprise two major apoptotic distinct pathways that converge on activation of caspases 19. Investigation regarding biomedical application of natural polysaccharides has recently attracted more attention 20. In the present study, novel polysaccharide was extracted from Persian Gulf brittle star O. erinaceus and its anticancer effect was evaluated. Moreover, this is the first study related to polysaccharide extracted from brittle stars which postulated therapeutic potency against cervical carcinoma. In this study, chromatin condensation, accumulation of sub-G1 followed by up-regulation of caspase-3 and caspase-9, down-regulation of Bcl-2 and up-regulation of Bax characterized apoptosis under treatment with brittle star crude polysaccharide were evaluated.
These findings are in agreement with other studies revealing apoptotic potential of natural polysaccharide in cancer therapy. In 2006, Lavi et al investigated in vitro anti cancer effect of partially isolated mushroom Pleurotus ostreatus polysaccharide fraction on HT-29 colon carcinoma cells and observed pro apoptotic effect via up-regulation of Bax and cytochrome C which may be candidate polysaccharide fraction in colon cancer medicine 21.
In 2010, Cao et al showed that polysaccharide isolated from Angelica sinensis exerted anti cancer effect via recruiting intrinsic apoptosis pathway in HeLa cervical cancer cells in vitro and in vivo 20. Chen et al. in 2013 studied chemopreventive properties of fungus extracted polysaccharide from Rhizopus nigricans and reported that the isolated polysaccharide suppressed human gastric cancer BGC-823 cell growth, displayed G2/M phase arrest and morphological indications of apoptosis through mitochondria mediated pathway 22. Also, fungus polysaccharide extracted from Armillaria mellea revealed a chemopreventive effect on A549 non-small cell lung cancer cells via increment of apoptosis and caspase activation through intrinsic pathway 23.
Chen et al in 2013 extracted water-soluble polysaccharide from roots of Dipsacus asperoides and showed that extracted polysaccharide inhibited osteosarcoma cell proliferation in a dose dependent manner with loss of mitochondrial potential and ROS accumulation that make it appropriate candidate against osteosarcoma 24.
Similar to natural polysaccharide extracted from marine sources, Gamal-Eldeen in 2009 evaluated anti proliferative and apoptotic effect of various fractions of polysaccharide isolated from brown algae (Sargassum latifolium). Their findings showed that E1 and E4 fractions induced DNA damage, increased macrophage proliferation and antimetastatic activity through anti-inflammatory capacity while E3 fraction indicated anti-cancer activity against 1301 leukemia cells 25.
Related to therapeutic capacity of marine invertebrate polysaccharide specially echinoderm, Nam et al in 2009 isolated crude polysaccharide from starfish (Asterina pectinifera) and assessed quinone reductase, glutathione S-transferase, omothine decarboxylase, cyclooxygenase and glutathione levels in HT-29 colon cancer cells and found that isolated polysaccharide attenuated omothine decarboxylase and cyclooxygenase and demonstrated protective effect of extracted polysaccharide against colon carcinoma14. Moreover, Lee et al in 2011, examined anti metastatic effect of polysaccharide extracted Asterina pectinifera on MDA-MB-231 breast cancer cells and confirmed that metastasis inhibitory effect of starfish polysaccharide can be considered potent anti tumor candidate in breast cancer 26. Lu et al in 2012 explored the tumor inhibitory effect of polysaccharide fraction from Coix lachrymal jobi (adlay seed) on A549 cancer cells and suggested that isolated polysaccharide triggers chemopreventive effect via involvement of intrinsic apoptotic pathway which can be appropriate in apoptosis targeted therapy 27.
In 2014, Wang et al evaluated the cytotoxic effect of Boschniakia rossica polysaccharide on Hep2 larynx squamous cells and their experiment showed the growth suppression, G0/G1 cell cycle arrest and apoptosis induction with up-regulation of Bax, DR5 and down-regulation of Bcl-2 28. Furthermore, Thangam et al studied tumor inhibitory effect of polysaccharide fractions from Cymbopogon citratus against LN-cap and Siha cancer cells and confirmed cytotoxic potential of natural extracted polysaccharide via apoptosis pathway 29. In conclusion, these experiments highlighted the importance of marine natural polysaccharides in anti cancer modalities.
Conclusion :
In this preliminary study, for the first time, Persian Gulf brittle star O. erinaceus polysaccharide was extracted and anti cancer potential of isolated polysaccharide was estimated on HeLa cervical carcinoma cells in vitro. Taken together, our experiment exhibited apoptosis inducing effect of brittle star extracted polysaccharide via mitochondria pathway which proposed alternative strategy in cervical cancer treatment.
Acknowledgement :
This work was performed in Research Center for Animal Development Applied Biology and was supported by the vice chancellor of scientific research of Mashhad Islamic Azad University. The authors have special thanks to the central lab of Mashhad medical university sciences.

Figure 1. Schematic depiction of polysaccharide extraction method from O. erinaceus.
|

Figure 2. A) Phenol-sulfuric acid reaction, production of furfural derivative characterized the carbohydrate content. (A=extracted polysaccharide,
B= blank, C=D-GLC). B) FTIR spectra in the 4000-400 cm_1 region of D-GLC and O. erinaceus which confirmed the similar peak of carbohydrates in extracted polysaccharide.
|
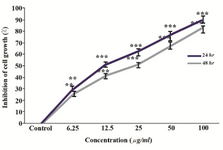
Figure 3. Evaluation of the cytotoxic effect of O. erinaceus crude polysaccharide on HeLa cells after 24 and 48 hr. Values are the mean±S.D. of triplicate determinations of three independent experiments **p<0.01, ***p<0.001.
|
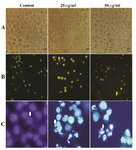
Figure 4. Morphological effect of O. erinaceus extracted polysaccharide on HeLa cells. Panel A) visualization with inverted microscopy. Panel B) AO/ PI double staining and Panel C) DAPI staining. Arrows in panel B from left to right point to live cells, apoptotic cells and necrotic cells and in panel C indicate chromatin condensation.
|

Figure 5. Estimation of apoptotic effect of O. erinaceus crude polysaccharide on cervical cancer cells by flow cytometry. Flow cytometry histogram of untreated and treated HeLa cells showed that inhibitory concentrations of extracted polysaccharide (25, 50 µg/ml) increased sub-G1 peak demonstrating involvement of apoptotic cells in cytotoxicity of brittle star polysaccharide.
|
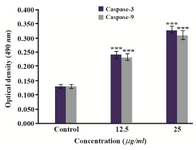
Figure 6. Treatment with O. erinaceus polysaccharide induced activation of caspase -3 and caspase -9 with increasing concentration of polysaccharide in HeLa cells.
***P<0.001 was considered significant between experimental groups and control.
|
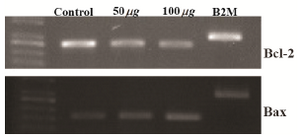
Figure 7. HeLa cells were treated with O. erinaceus extracted polysaccharide and the mRNA expression of Bax and Bcl-2 were assessed. RT-PCR analysis indicated that isolated polysaccharide exerted pro apoptotic effect on HeLa cells.
|
|