An Investigation on Mitochondrial DNA Deletions and Telomere Shortening during Multiple Passages of Adult Stem Cells
-
Fesahat, Farzaneh
-
Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
-
 Sheikhha, Mohammad Hasan
Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran, Tel: +98 351 8247085; Email:sheikhha@yahoo.com
Sheikhha, Mohammad Hasan
Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran, Tel: +98 351 8247085; Email:sheikhha@yahoo.com
-
Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
-
Rasti, Azam
-
Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
-
Zare-Zardini, Hadi
-
Young Researchers and Elite Club, Yazd Branch, Islamic Azad University, Yazd, Iran
-
Navabazam, Ali Reza
-
Department of Oral and Maxillo-facial Surgery, School of Dentistry, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Abstract: Background: Limited resources for adult stem cells necessitate their in vitro culture prior to clinical use. Investigating mitochondrial DNA (mtDNA) and telomere shortening has proved to be important indications of stem cell validity. This study was designed to investigate these indicators in multiple passages of three adult stem cell lines which were produced in our stem cell laboratory.
Methods: In this study, Dental Pulp Stem Cells (DPSCs), Periapical Follicle Stem Cells (PAFSCs) and Human Foreskin Fibroblast (HFF) cell lines were expanded for 20 passages. After 1, 5, 10, 15 and 20 passages, expanded cells were harvested and DNA was extracted for further studies. Common mtDNA mutation was detected by multiplex PCR and telomere shortening was tested by Southern blot analysis.
Results: The common deletion was not detected in any of the stem cells or cell lines after several passages. In addition, Southern blot analysis indicated that the mean difference of telomere length between first and last passage was 0.25 kb in DPSC, 0.1 kb in PAFSC and 0.32 kb in HFF which indicates that the mean telomere length in various passages of the samples showed insignificant changes.
Conclusion: Absence of mtDNA mutations in adult stem cell lines indicates good mitochondrial function even after 20 passages. In addition, absence of telomere shortening indicates stem cells validity after multiple passages. It is hoped this information could pave the way for using in vitro expansion of adult stem cells for future clinical applications.
Introduction :
Stem cell therapy is a new method for the treatment of many different diseases. Recently, the stem cells originating from adult tissues have been used for this purpose. Limited resources for these kinds of stem cells necessitate their in vitro culture prior to clinical use. The researchers use some indicators in the cell lines to investigate whether expanded adult stem cells have a long enough life span for future division in vivo or not.
Mitochondria play a vital role throughout the life cycle of the eukaryotic cells. It is involved in many metabolic tasks such as apoptosis-programmed cell death and cellular proliferation 1. One fundamental function of mitochondria is the production of Adenosine Triphopshate (ATP) via oxidative phosphorylation. This could be disrupted when a critical threshold of mutant mitochondrial DNA (mtDNA) is met, thus inactivating the cell 2. Therefore, the evaluation of common mtDNA deletion could be used as a marker to detect the quality of adult stem cells. Numerous laboratories have detected a specific mtDNA mutation, known as the common deletion (ΔmtDNA4977). This mutation is associated with some neoplasms 3,4 and also aging processes in some tissues and cells 5-10. Adult cell lines may harbor mutant mtDNA that may further accumulate during cell culture, and thus have consequences for long-term viability. On the other hand, future medical applications of stem cell therapies include the use of cells originating not only from embryos but also from adult tissues 11. Usually, it is essential to culture and populate adult stem cell line or other adult cell sources like fibroblasts before putting them to therapeutic use. Therefore, monitoring the volubility of these cells after multiple cultures is very important.
In addition to mutant mtDNA, telomere shortening is another good indicator of stem cells volubility. There are several methods for monitoring or measuring the condition of telomeres such as Terminal Restriction Fragment (TRF) analysis, Fluorescence in situ Hybridization (FISH), Polymerase Chain Reaction (PCR) and telomerase activity 12. These measurements are important because extensive sub-culturing and expansion of adult stem cells is necessary for an effective therapy 13.
In this paper, adult stem cell lines were established from human third molar teeth and cell lines from human foreskin fibroblast in Yazd Research and Clinical Center for Infertility 14,15. The goal of this study was to determine whether the human mtDNA common deletion and shortening of telomere length as two aging biomarkers were present in these cell lines during several passages.
Materials and Methods :
Isolation of stem cells and cell culture: The normal and non-decayed human third molars were extracted for orthodontic treatment purposes from 20 adults (15-22 yrs) after receiving their informed consent at the Department of Oral and Maxillo-Facial Surgery, Dental School of Shahid Sadoughi University of Medical Sciences, Yazd, Iran 14. Dental School Ethics Committee approved the research and all the participants submitted their signed consent letter.
The pulp tissue was gently separated from the crown and root. Tooth germs at the root-forming stage were obtained and named periapical follicles. These were removed from the root dentin with a scalpel. The tissue segments were digested in a solution of 3 mg/ml collagenase type I and 4 mg/ml dispase dissolved in DMEM for 1 hr in CO2 incubator. The suspensions of cells were cultured into 25 cm2 plastic flask with alpha modification of Eagle’s medium (alpha-MEM, Gibco BRL, Carlsbad, CA) supplemented with 10% Fetal Bovine Serum (FBS, Gibco BRL), 100 U/ml penicillin, 100 mg/ml streptomycin (Gibco BRL) and amphotericin B (BIOCHROM AG) for primary culture and then incubated at 37oC. In this study, 2 types of derived dental stem cells, DPSCs and PAFSCs were passaged 21 times and after each passage 3 to passage 21, mtDNA deletion was investigated.
Isolation of human foreskin fibroblast: Pieces of human foreskin with the area of 1-2 cm2 were obtained from circumcisions of 9 human neonatas. These pieces were washed with phosphate saline buffer solution (Invitrogen Corporation, Carlsbad, CA), then they were cut into 4-6 mm pieces, washed again, and incubated enzymatically by collagenase I and IV with a 1:1 ratio (Invitrogen Corporation, Carlsbad, CA) overnight at 37°C. The tissue pieces were fragmentized mechanically with insulin syringes (29 gauge), and then cell suspension was centrifuged at 1,000 rpm for 5 min and the supernatant was removed. Next, the pellet was resuspended in 10 ml proliferation DMEM medium supplemented with 15% FBS and 1% penicillin/streptomycin, then transferred to a 25 cm2 tissue culture flask (Falcon) 15. Multiple passages were performed for Human Foreskin Fibroblast (HFF) and passages 1, 5, 10, 15 and 20 were used for comparing them with two adult dental stem cell lines in the study.
DNA extraction and mtDNA deletion analysis: Using Bioneer Genomic DNA kit, total cellular DNA was isolated from different cell lines in passages 1, 5, 10, 15 and 20. Each experiment was repeated three times for all passages. The PCR reactions were performed in a thermal cycler (MWG biotech primus) for 35 cycles with denaturation at 94oC for 1 min, primer annealing at 55oC for 1 min and extension at 72oC for 35 s. The deletion- prone region of mtDNA between 5461 of light strand and 15000 of heavy strand was investigated in all samples using the primers ONP 86 (5461-5480), ONP 89 (5740-5721), ONP 74 (13640-13621), ONP 25 (8161-8180) 16. The distances between the primers were long enough to allow amplification only if a part of the DNA between respective primers was deleted. Primer pairs, ONP 86 and ONP 89, were used to amplify a normal internal mtDNA fragment (279 bp) in a region which is rarely afflicted by deletions, and served as a control for the preparation and PCR analysis. Primer pairs, ONP 74 and ONP 25 were used to amplify a 502 bp amplicon for detection of deletion. One DNA sample from a patient with Ataxia-Telangiectasia who had a common mtDNA deletion was tested as the positive control. The accuracy of tests was examined by direct sequencing of some random DNA fragments amplified by the PCR reactions.
Southern blot analysis of TRF: Telomere restriction fragment analysis was performed as described by using the TeloTAGGG Telomere Length Assay Kit (Roche Molecular Biochemical) according to the protocol provided by the manufacturer's recommendations. Briefly, the test principle is as follows: The genomic DNA was isolated and digested using restriction enzymes RsaI and HinfI for 2 hr at 37°C. The DNA fragments were separated by gel electrophoresis for 2-4 hr at 70 V on a 0.8% agarose gel and transferred to a nylon membrane by Southern blotting. The blotted DNA fragments were hybridized to a digoxigenin (DIG)-labeled probe specific for telomeric repeats and incubated with a DIG-specific antibody covalently coupled to alkaline phosphate. Finally, the immobilized telomere probe was visualized by virtue of alkaline phosphatase metabolizing CDP-Star, a highly sensitive chemiluminescence substrate. The average TRF length could be determined by comparing the signals relative to a molecular weight standard. After exposure of the blot to an X-ray film, the mean TRF length were scanned and calculated by the multianalizor (Bio-rad) software.
Statistics: All values are expressed as means±SD. Differences between groups were tested using paired t-test. A p-value<0.05 was considered significant.
Results :
mtDNA common deletion: In this study, using a molecular approach, the frequency of the mtDNA common deletion was analyzed in adult cell lines derived from dental and foreskin tissues. The common deletion was not found in any samples (Figure 1). The results demonstrated that the mtDNA common deletion was unlikely to be responsible for aging due to multiple routine passages.
Telomere length of adult stem cells: After three experiments, the mean telomere length in the first passage was 11.86±0.55 kb in DPSC, 11.47±0.15 kb in PAFSC and 11.51±0.14 kb in HFF. Moreover, mean telomere length in the last passage was 11.61±0.58 kb in DPSC, 11.57±0.28 kb in PAFSC and 11.19±0.17 kb in HFF (Figure 2). These results show that the mean difference of telomere length between first and last passage was 0.25 kb in DPSC, 0.1 kb in PAFSC and 0.32 kb in HFF which indicate insignificant changes with p-values greater than 0.05 (Table 1). In addition, morphological investigation by microscope and cell proliferation rate assay for achieving cell density did not show appreciable changes during passages (Table 2).
Discussion :
Investigating mtDNA common mutation and telomere length assay as aging biomarkers of adult stem cells is the subject of many recent studies. For example, several studies were done in this regard on Mesenchymal Stem Cell (MSC) lines derived from human bone marrow 12-14, 17 or animal 9 and also on oocytes, embryo or cumulus cells 18,19. In this study, efforts were made to examine mtDNA common mutation and telomere length assay together in adult human dental stem cells and adult fibroblast line which were produced in our stem cell laboratory during multiple passages.
Adult stem cells including MSCs and Hematopoetic Stem Cells (HSCs) are generally known to have a limited life span 20. Human Mesenchymal Stem Cells (hMSCs), because of their self renewal ability, multipotentiality, and immunosuppressive capacity are considered to have high clinical potentials. These potentials could be different depending on tissue origin and some embryonic stem cell like phenotypes such as expression of embryonic markers 12,21.
Some studies introduced adult stem cell lines with detectable levels of telomerase that can extend to a large population at multiple passages in vitro 22. Moreover, the mechanism of senescence for these cell lines is poorly understood. Replicative senescence proceeded and it was shown that stress-induced premature senescence, unlike replicative senescence, is largely independent of the telomere length or the number of cell divisions 20. Chen et al indicated that Amniotic Fluid (AF) stem cells show senescence and longevity changes unrelated to telomere length. Therefore, AF derived cells are most likely to undergo stress induced premature senescence and not normal replicative senescence in culture 23.
In adults, telomerase is only expressed in germ cells, stem cells and some progenitor cells 24 while presence of telomerase activity in MSCs is still controversial according to different studies 25-32.
It is reported that the longest telomeres are generally marking the most primitive adult stem cell compartments and the shortest telomeres are related to more differentiated compartments within a given tissue 25. Similar results were reported in lingual epithelium 26. In order to better explain the role of telomeres in the aging of MSCs, initial size of telomeres and donor age dependency were examined. Interestingly, the data from different groups do not correlate with each other 28-30. However, findings related to the gradual deterioration of telomere ends with successive cell divisions are consistent across all studies 12.
In our previous work on PAFSCs, DPSCs and HFF, high proliferative ability of these cell lines was shown by the expression of some embryonic/germ immonohistochemical or gene markers which indicate that their self renewal capacities may increase without occurrence of aging markers 14,15. Overall, some investigators used cancer stem cells instead of adult stem cells 33. Studying this population is easier for monitoring their behavior in response to different stimuli or therapeutic treatments 34. However, in this study, generated stem cells plus HFF cell line were used which are the main strengths of this research.
In the present study, an insignificant change of telomere length was observed between first and last investigated passages in all three cell lines. In addition, morphological investigation by microscope and cell proliferation rate assay for achieving cell density did not show appreciable changes during passages.
In addition to the telomere length, the accumulation of common deletion as well as other mtDNA deletions and point mutations are thought to contribute to normal cell aging 27. In the past decade, different mutations of mtDNA have been reported to frequently occur and accumulate with age in muscle and other human tissues. Among them, the 4,977 bp and 7,436 bp deletions and the A3243G and A8344G point mutations are the most frequent mutations 35. In fact, the accumulation of mtDNA deletions and Single Nucleotide Variants (SNVs) is a well-accepted facet of the biology of aging. Recently, it was shown that a complex manner is involved in alteration of the mitochondrial genome with age 36. It was shown that mitochondria are translocated in the oocyte during fertilization to cluster around the pronuclei and remain in a perinuclear pattern during embryo development. This clustering appears to be essential for normal embryonic development. Because embryonic stem cells are derived from fertilized oocytes, and eventually can differentiate into adult stem cells, it was hypothesized that mitochondrial perinuclear clustering persists through preimplantation embryo development into the stem cells, and that this localization is indicative of stem cell pluripotency 7,19.
The study did not reveal a specific association between common 4977 bp mtDNA deletion and multiple passages of adult stem cells and fibroblasts that were used as a common cell line. Similar to this study, several other studies using different tissue/cell lines had already come to the same conclusion 3.
Conclusion :
With these results, it seems that common mtDNA deletion and telomere length are not related with aging in 20 first passages in adult stem cell lines and HFF cell line. Therefore, these adult stem cells may be suitable for clinical application even after 20 passages. This article suggests further investigation on different adult cell lines with more passages in vitro for mtDNA deletion and telomere length assay. This will expand our data regarding the effect of multiple passages and cellular senescence of stem cell lines on gene expression changes in adult stem cells.
Conflict of Interest :
There were no conflicts of interest to be stated.
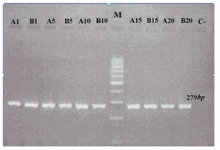
Figure 1. Gel electrophoresis to study human mitochondrial DNA (mtDNA) common deletion in adult stem cell lines (DPSCs). Marker is a 100 bp DNA ladder. The other lanes demonstrate 279 bp mtDNA amplified with primers ONP 86 and 89. No PCR amplicons were generated in any three cell lines using primers ONP 74, ONP 25, ONP 99, ONP 10 indicating no mtDNA common deletion. A1, A5, A10, A15 and A20 indicate periapical follicle stem cells (PAFSCs) after 1, 5, 10, 15 and 20 passages. B1 B5, B10, B15, B20 indicate the same results in dental pulp stem cells (DPSCs). C and M show negative control and marker, respectively
|

Figure 2. Southern blotting shows telomere length. A5, A10, A15 and A20 indicate telomere length in PAFSCs after 5, 10, 15 and 20 passages. B5, B10, B15, B20 and C5, C10, C15, C20 indicate the same results in DPSCs and HFF, respectively. M is molecular weight marker. LW and HW indicate low and high molecular weight of control DNA, respectively
|
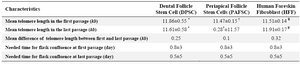
Table 1. Characteristics of dental stem cell lines and human foreskin fibroblasts
Comparisons of groups *†¥ with mean telomere length in the last passage were not statistically significant. P>0.05
|
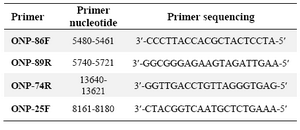
Table 2. Primer used for common deletion detection
|
|