Green Synthesis of Small Silver Nanoparticles Using Geraniol and Its Cytotoxicity against Fibrosarcoma-Wehi 164
-
Safaepour, Mona
-
Department of Pharmaceutical Biotechnology and Biotechnology Research center, Faculty of Pharmacy, Tehran University of Medical Sciences , Tehran, Iran
-
 Shahverdi, Ahmad Reza
Ph.D., Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Tehran University of Medical Sciences, P.O. Box: 14155-6451, Tel: +98 21 66959090, Fax: +98 21 66482706, E-mail: shahverd@sina.tums.ac.ir
Shahverdi, Ahmad Reza
Ph.D., Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Tehran University of Medical Sciences, P.O. Box: 14155-6451, Tel: +98 21 66959090, Fax: +98 21 66482706, E-mail: shahverd@sina.tums.ac.ir
-
Department of Pharmaceutical Biotechnology and Biotechnology Research center, Faculty of Pharmacy, Tehran University of Medical Sciences , Tehran, Iran
-
Shahverdi, Hamid Reza
-
Department of Material Science, Faculty of Engineering, Tarbiat Modares University , Tehran, Iran
-
Gohari, Ahmad Reza
-
Medicinal Plants Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences , Tehran, Iran
Abstract: Many reports have been published about the biogenesis of silver nanoparticles using several plant extracts such as Pelargonium graveolens (P.graveolens- geranium) and Azadirachta indica (neem) but the capacity of their natural reducing constituents to form silver nanoparticles has not yet been studied. In this research the synthesis of silver nanoparticles using geraniol has been investigated. We successfully synthesized uniformly dispersed silver nanoparticles with a uniform size and shape in the range of 1 to 10 nm with an average size of 6 nm. Also the cytotoxicity of the prepared silver nanoparticles was investigated using a cancer cell line (Fibrosarcoma-Wehi 164). The cytotoxicity analysis of the sample shows a direct dose-response relationship; cytotoxicity increased at higher concentrations. At concentration of 1 µg/ml, silver nanoparticles was able to inhibit the cell line’s growth by less than 30%. Conversly, the presence of 5 µg/ml of silver nanoparticlse significantly inhibited the cell line’s growth (> 60%). The concentration necessary to produce 50% cell death was 2.6 µg/ml for this silver nanoparticles preapared with geraniol.
Introduction :
The development of green processes for the synthesis of nanoparticles is evolving into an important branch of nanotechnology (1, 2).
Today, nanometal particles, especially silver, have drawn the attention of scientists because of their extensive application in the development of new technologies in the areas of electronics, material sciences and medicine at the nanoscale (3-5). Silver nanoparticles have many applications; for example, they might be used as spectrally selective coatings for solar energy absorption and intercalation material for electrical batteries, as optical receptors, as catalysts in chemical reactions, for biolabelling, and as antimicrobials. (3, 4, 6). Many reports have been published in the literature on the biogenesis of silver nano-particles using several plant extracts, particularly Neem leaf broth (Azadirachta indica) and geranium leaves (P. graveolens) (7, 8). The reducing property of different plant constituents such as geraniol (Figure 1) may play a critical role in the reduction of Ag+ to silver nanoparticles (8). However, the synthesis of silver nanoparticles using plant constituents has not yet been studied for a large number of natural compounds. In this study, the synthe-sis of silver nanoparticles using geraniol has been investigated. Also, in this study the cyto-toxicity of silver nanoparticles prepared by geraniol was investigated using a cancer cell line (Fibrosarcoma-Wehi 164).
Materials and Methods :
Synthesis and characterization of silver nano-particles
Aqueous solution containing Ag+ ions (1 mM) are prepared by adding 100 µl of oily geraniol (Carol Roth GmbH+Co, Karlsruhe, Germany) and 10 ml of 1 %w/v aqueous solution of polyethylene glycol 4000 (Merck, Germany) to 90 ml of silver nitrate solution. This was then alkalized with 0.1 NaOH (20 µl) and treated in a microwave oven (850 W) for 40 sec for the reduction of metal ion. In a series of parallel experiments, the reaction takes place at room temperature. The reduction of the Ag+ ions by geraniol in the solutions was monitored by sampling the aqueous component (2 ml) and measuring the UV–visible spectrum of the solutions. UV–visible spectra of these aqueous colloid samples (1 mM) were measured on a Labo-med Model UVD-2950 UV-VIS Double Beam PC Scanning spectrophotometer, oper-ated at a resolution of 2 nm. Furthermore, silver nanoparticles were characterized by transmission electron microscopy (CM 200 FEG, Philips) and energy-dispersive spectros-copy (EDS).
Cell culture and cytotoxicity assay
The Fibrosarcoma cell line (Wehi 164) was seeded in 96-well tissue culture plates. Cells were maintained in a RPMI-1640 medium that was supplemented with 5% fetal calf serum plus antibiotics at 5% CO2, 37 oC, and saturated humidity. The Fibrosarcoma-Wehi 164 cell line was obtained from the National Cell Bank of Iran (NCBI), Pasteur Institute of Iran, Tehran (Iran). Colloidal Ag-NPs solu-tion (10 mg/ml) was centrifuged at 18000 rpm for 1 hr and sediment was re-suspended in distilled water. Triplicate, different concentra-tions of silver nanoparticles (1, 2, 3, 4 and 5 µg/ml) were transferred to overnight cultured cells. Non-treated cells were used as control. Cells were cultured overnight and were then subjected to Crystal Violet colori-metric assay. Cytotoxicity was expressed as the percentage of viable cells at different con-centrations of samples. IC 50 was calculated as the dose at which 50% cell death occurred relative to the untreated cells.
In the cytotoxicity assay, cells in the exponential phase of growth were incubated for 24 hr at 37 oC with 5% CO2 with different concentrations of silver nanoparticles. The cell proliferation was evaluated by a modified Crystal Violet colorimetric assay (9). After each experiment, the cells were washed with ice-cold phosphate buffer solution and fixated in a 5% formaldehyde solution. Fixed cells were stained with 1% crystal violet. Stained cells were lysed and solubilized with a 33.3% acetic acid solution. The density of developed purple color was read at 580 nm. The differ-ences in cell cytotoxicity were compared using the Student’s t test. P values <0.05 were considered significant.
Result :
Synthesis of silver nanoparticles
The chemical reduction of aqueous solution of silver nitrate is one of the most widely used methods for the synthesis of silver colloids. In this study, the formation of silver nanopar-ticles by geraniol was investigated. The appearance of a yellowish brown color in the reaction vessels suggested the formation of silver nanoparticles (10). Figure 2 shows the bottles containing the silver nitrate (1 mM) before (tube A) and after reaction with geraniol for 40 sec under heating in micro-wave oven (tube B). Also, no color change was observed when the procedure took place at room temperature or stayed for 24 hr in the same conditions (Figure not shown). These reaction mixtures were further characterized by UV-visible spectroscopy.
The technique outlined above proved to be very useful for the analysis of nanoparticles (11, 12). As illustrated in Figure 3, a strong, broad absorption band with a maxima located at 440 nm was observed due to formation of silver nanoparticles produced by the geraniol. This peak is assigned to a surface plasmon, phenomenon that is well-documented for various metal nanoparticles with sizes ranging from 2 nm to 100 nm (11, 12).
Particle size and its chemical composition
Figure 4 shows representative TEM images recorded from the drop-coated film of the silver nanoparticles synthesized by treating the silver nanoparticles solution with the geraniol. The particle size histogram of silver particles produced by geraniol (right illustra-tion in Figure 4) shows that the particles range in size from 1 nm to 10 nm, and possess an average size of 6 nm. In the analysis of the silver nanoparticles by Energy Dispersive Spectroscopy (EDS), the presence of elemen-tal silver signal was confirmed in the sample (Figure 5). The Ag nanocrystallites display an optical absorption band peaking at 3 keV which is typical of the absorption of metallic silver nanocrystallites (5).
Cytotoxicity of silver nanoparticles
The cytotoxicity of the silver nanoparticles was evaluated in vitro against Fibrosarcoma-Wehi 164 at different concentrations (1, 2, 3, 4, 5 µg/ml). Our cytotoxicity analysis of the sample shows a direct dose-response relation-ship; cytotoxicity increased at higher concen-trations (Figure 6). The samples demonstrated a considerable cytotoxicity against the Fibro-sarcoma-Wehi 164. The concentration neces-sary to produce 50% cell death was 2.6 µg/ml for the silver nanoparticles. As shown in Figure 1, in the lowest tested concentration (1 µg/ml), silver nanoparticles were able to inhibit the cell line’s growth by less than 30%. In contrast the presence of 5 µg/ml of silver nanoparticles significantly inhibited the cell line’s growth (> 60%).
Discussion :
The potential ability of geraniol for the reduction of Ag+ to silver nanoparticles was investigated in different condition. Characteri-zation by UV-visible, TEM and EDS tech-niques confirmed the reduction of silver ions to silver nanoparticles. To the best of our knowledge, and based on a thorough literature surveys, this is the first report on the synthesis of silver nanoparticles using geraniol as a volatile compound from different plants such as Pelargonium graveolens (geranium).
Also, in this investigation, a cytotoxicity assay was used to assess the effect of silver nanoparticles on the proliferation of a cancer cell line. No studies have been conducted, however, on the cytotoxicity of silver nano-particles against Fibrosarcoma-Wehi 164. This is the first study of the cytotoxicity of silver nanoparticles against Fibrosarcoma-Wehi 164. The silver nanoparticles prepared by geraniol showed significant cytotoxicity against Fibrosarcoma-Wehi 164 cell line. Only 2.6 µg/ml silver nanoparticles was necessary to decrease cell proliferation by 50%.
Acknowledgement :
This work was financially supported by the Medicinal Plants Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran and by Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
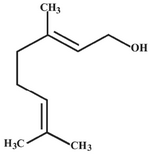
Figure 1. Chemical structure of geraniol
|
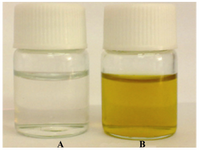
Figure 2. Solutions of silver nitrate (1 mM) before (A) and after exposure to the geraniol (B) heated in microwave oven (850 W) for 40 sec
|
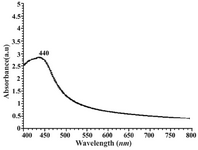
Figure 3. UV-visible spectrum of aqueous silver colloid (1mM) prepared by geraniol
|
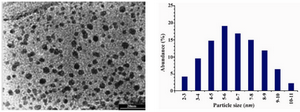
Figure 4. Transmission electron micrographs recorded from a small region of a drop-coated film of silver nitrate solution treated with the geraniol (left picture) for 40 sec in a microwave oven (scale bars correspond to 20 nm). The related particle size distribution histograms (right picture) was obtained after counting 300 individual particles
|
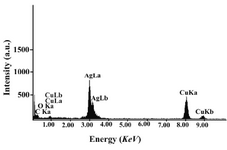
Figure 5. EDS spectra of prepared silver nanoparticles. Silver X-ray emission peaks are labeled. Strong signals from the atoms in the nanoparticles are observed in spectrum and confirm the reduction of silver ions to silver nanoparticles
|
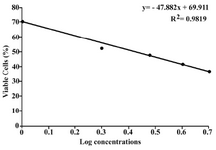
Figure 6. Cytotoxicity of silver nanoparticles prepared by geraniol against Fibrosarcoma Wehi-164. Standard devi-ations for each concentration were negligible
|
|