Messenger RNA Expression Patterns of Neurotrophins during Transdifferentiation of Stem Cells from Human-Exfoliated Deciduous Teeth into Neural-like Cells
-
 Esmaeili, Abolghasem
Cell and Molecular Biology Division, Department of Biology, School of Sciences, University of Isfahan, Isfahan, Iran, Tel: +98 311 732490; Email: aesmaeili@sci.ui.ac.ir
Esmaeili, Abolghasem
Cell and Molecular Biology Division, Department of Biology, School of Sciences, University of Isfahan, Isfahan, Iran, Tel: +98 311 732490; Email: aesmaeili@sci.ui.ac.ir
-
Cell and Molecular Biology Division, Department of Biology, School of Sciences, University of Isfahan, Isfahan, Iran
-
Alifarja, Sedigheh
-
Cell and Molecular Biology Division, Department of Biology, School of Sciences, University of Isfahan, Isfahan, Iran
-
Nourbakhsh, Nosrat
-
School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran
-
Torabinejad Dental Research Center, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran
-
Talebi, Ardeshir
-
Department of Pathology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Abstract: Background: Stem cells from Human Exfoliated Deciduous teeth (SHED) have the capability to differentiate into neural cells. Neurotrophins including Nerve Growth Factor (NGF), Brain-Derived Neurotrophic Factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4) have neurogenesis, neurotrophic, or neuroprotective effects and are expressed in developing teeth. The aim of this study was to measure quantitative changes in mRNA expression levels of neurotrophins in neural-like cells differentiated from dental pulp stem cells.
Methods: Isolated total RNA from SHED, dental pulp and neural-like cells (n=3) were transcribed into cDNA. Then real time PCR was done. Expression levels of mRNA for NGF, BDNF, NT-3, and NT-4 genes were compared in these three cells.
Results: In neural like cells, BDNF mRNA increased (372.1113.5) significantly (p<0.01) after differentiation. NGF mRNA increased to more than 266 times the dental pulp level after differentiation. A similar pattern was seen for the expression of NT3 after differentiation. NT4 mRNA enhancement was 1344630.8 and 30.77.9 fold in neural like cells and SHED cells, respectively. Results show alterations with different degrees and direction in neurotrophins mRNA expression levels in these cells.
Conclusion: Our results suggest that neurotrophins dental pulp cells, SHED cells and neural like cells derived from SHED cells produce neurotrophic factors. Since the large amounts of neurotrophins are expressed in SHED and neural like cells they may have important role in survival and differentiation of dental pulp stem cells and obtained information may lead to a novel method for tooth regeneration.
Introduction :
Regeneration ability of the adult mammalian Central Nervous System (CNS) is limited after injury or disease. Currently, effective strategies are required for treating patients suffering from CNS injuries or diseases. Strategies such as incorporating the sustained release of neurotrophic factors, gene therapy and cell therapy have been suggested for their ability to overcome this limited regeneration, but may face clinical limitations such as volume restrictions and the inability of donor cells to interact with the host tissue 1, 2.
The isolation and characterization of neural stem/progenitor cells from different tissues with subsequent direct cell transplantation of these cells into the damaged brain is one important approach for cell therapy. This is because of multipotential ability of these cells to renew themselves and give rise to neurons, astrocytes and oligodendrocytes.
Neural stem/progenitor stem cells have been isolated from various tissues, including bone marrow, neural tissue, skin, retina, and dental epithelium 3-8. Incidentally, autologous stem/progenitor cells can be collected from dental tissue including Dental Pulp Stem Cells (DPSCs), SHED, Periodontal Ligament Stem Cells (PDLSCs), Stem Cells from Apical Papilla (SCAP), and Dental Follicle Progenitor Cells (DFPCs) 9. Achieving SHED is easy and convenient, with little or no trauma. They have the capability to differentiate into osteoblasts, odontoblasts, endothelial cells, adipocytes, and neural cells 10-13.
It has been demonstrated that neurotrophins including NGF, BDNF, NT-3, and NT-4, (also known as NT-5) have neurogenesis, neurotrophic, or neuroprotective effects on the brain. Neurotrophins are expressed in developing teeth 14-20. Neurotrophins may have important role in differentiation of SHED into neural-like cells. We compared mRNA expression levels of NGF, BDNF, NT3 and NT4 genes, dental pulp, SHED and neural-like cells differentiated from SHED.
Materials and Methods :
Isolation of dental pulp: In this study, 12 normal human exfoliated deciduous incisors were collected from six to nine year-old children under approved guidelines set by Isfahan University of Medical Sciences, Faculty of Dentistry. The pulps were separated from the crown and harvested in a lysis buffer (Qiagen, Hilden, Germany) and stored at -80C for RNA extraction.
Cell culture: SHED were purchased from Royan Institute and subcultured as previously described 21. Briefly, cells were thawed and resuspended in DMEM/F12 supplemented with 10% FCS. Upon reaching 80-90% confluence, cells were either harvested for phenotypic characterization and differentiation or stored at -80°C for total RNA isolation at later times.
Neural Differentiation: Neural differentiation was conducted with protocol published previously 21. Briefly, SHED at passage 4 were seeded on poly-L-lysine (Sigma, P4707)-coated glass cover slips at a concentration of 5000 cells/cm2 in neurobasal medium containing 1% ITS (Invitrogen, 41400), and cytokines including 100 ng/ml basic fibroblast growth factor (bFGF, Sigma, F0291) for seven days followed by 100 ng/ml bFGF, 10 ng/ml FGF8 (Sigma, F6926), and 100 ng/ml sonic hedgehog (SHH, Sigma, S0191) for an additional seven days. During the first seven days of induction, media were half refreshed and bFGF was added with the full concentration. Following media exchange on day seven, the culture was continued without any media renewal. Differentiated cells were stored at −80°C for total RNA isolation at later times.
RNA extraction and reverse transcription (RT): Total cellular RNA was isolated from frozen tissues using the RNeasy Mini kit (Qiagen). The extracted RNA was dissolved in Diethyl Pyrocarbonate (DEPC)-treated water. The purity and integrity of the extracted RNA were evaluated by optical density measurements (260/280 nm ratios) and by visual observation of samples electrophoresed on agarose gels. Both methods indicated integrity of the extracted RNA with little or no protein contamination. Complementary DNA synthesis reactions were performed using 1 µg DNase (Fermentas)-treated total RNA from each sample and cDNA synthesis kit (Fermentas) with random hexamer (Fermentas) priming in a 20 µl reaction according to the manufacturer’s instructions.
Real-time PCR: Real-time PCR was performed in the Chromo4 Detection System (BioRad, USA). Briefly, 25 ng of cDNA and gene specific primers were added to SYBR green master kit (Takara, Tokyo, Japan), and subjected to PCR amplification (1 cycle at 95C for 10 min, and 45 cycles at 94C for 5 s, 59C for 20 s and 72C for 15 s). All PCR reactions were run in triplicate.
Gene-specific primers were designed by Beacon Designer 7.5 software. The primers used for real time PCR are listed in table 1. Expression levels were normalized to that of actin. Relative expression data was quantified using 2- ΔΔCt where Ct is the cycle threshold. Relative standard curves were generated by plotting the threshold value (Ct) versus the log of the amount of total cDNA added to the reaction and used to check the efficiency of primers. Calculation of Ct, standard curve preparation and quantification of mRNA in the samples were performed by the software provided by Chromo4 (Option 3). All target genes were normalized to the actin housekeeping gene.
Statistical analysis: To determine significance, all data were subjected to statistical analysis using a computerized statistics program (GraphPad Prism). One-way ANOVA was used, as indicated in the figure legends, followed by a post hoc test (Tukey) of differences between specific time points. All data were presented as the means ±S.E.M. A level of p<0.05 was considered significant.
Results :
BDNF, NGF, NT3 and NT4 genes showed significant alterations in mRNA expression after differentiation of SHED into neural-like cells. Figure 1 shows changes in mRNA expression of BDNF, NGF, NT3 and NT4 in dental pulp tissue, SHED and neural-like cells. In each case, the ratio of the obtained data to that expressed in the dental pulp tissue was indicated.
Alterations in BDNF expression after differentiation: BDNF expression was altered after differentiation (Figure 1A). One-way ANOVA indicated significant alteration after differentiation. In SHED, BDNF mRNA increased 107.4±34.35 fold which was not significant (p>0.05) (Figure 1A). In neural-like cells, BDNF mRNA began to increase (372.1±113.5) after differentiation. Enhancement at these cells was significant (p<0.01) (Figure 1A). When BDNF expression levels of SHED were compared with neural-like cells, there was a significant difference between them (p<0.05) (Figure 1A).
Alterations in NGF expression after differentiation: In contrast, NGF mRNA (Figure 1B) was higher than dental pulp in both SHED and neural-like cells. The greatest alterations were seen in neural-like cells where NGF mRNA increase was 266 times higher than the dental pulp level after differentiation. SHED showed a 130.8±31.28 fold increase in mRNA (Figure 1B). In neural-like cells, the mRNA expression level of NGF was significantly (p<0.05) more than the SHED level.
Alterations in NT3 expression after differentiation: A similar pattern was seen for the expression of NT3 after differentiation (Figure 1C). NT3 mRNA significantly (p<0.001) increased by 1375±259.6 and 338.8±82.13 fold in neural-like cells and SHED, respectively.
Alterations in NT4 expression after differentiation: NT4 mRNA significantly (p<0.05) increased in neural-like cells when compared with both dental pulp tissue and SHED. NT4 mRNA enhancement was 1344±630.8 and 30.7±7.9 fold in neural-like cells and SHED, respectively (Figure 1D).
Discussion :
We analyzed the expression of the mRNA for BDNF, NGF, NT3 and NT4 genes after differentiation of SHED into neural-like cells.
In the current study, SHED were differentiated towards neural phenotype with a previously described protocol 21. Real-time PCR allowed us to obtain quantitative data before and after differentiation with respect to the dental pulp tissue. Each of the BDNF, NGF, NT3 and NT4 genes appeared to be unique in its pattern of alterations after differentiation. To our knowledge, this is the first time that mRNA expression levels after differentiation of SHED into neural-like cells for BDNF, NGF, NT3 and NT4 genes were shown.
We supposed that when SHED differentiated into neural cells, neurotrophins mRNA expression levels may change. Neurotrophic factors and their receptors were expressed during tooth development. This implies their important functions in odontogenic processes. Neurotrophic factors derived from dental pulp play an important role in orchestrating the dental pulp innervations 22. This suggests that these neurotrophic factors might be involved in the innervation of dental structures. The rich expression of neurotrophic factors in developing dental tissues proposes that developing, or possibly adult, dental tissue might be used as an allograft source of trophic support for diseases of the nervous system such Alzheimer’s disease 23. Neurotrophic factors derived from dental pulp may also act as target-derived neurotrophic factors in the trigeminal system. These factors promote the survival of dopamine (DA) neuron and provide neuroprotection for DA neurons. Some dental pulp cells also differentiate into cells with neuronal characteristics 24.
It has been shown that NT-4 regulates proliferation and differentiation of dental epithelium, and promotes the production of enamel matrixes 25. Results of another study revealed that all neurotrophin mRNAs were detected in embryonic teeth 18. They also showed the developmentally changing, distinct expression patterns for NGF and NT-3. It has been shown that NGF is involved in the guidance of trigeminal axons to embryonic teeth. Expression of NGF mRNAs in postnatal teeth was correlated with trigeminal axon growth. This may propose a role for NGF in local sprouting and establishment of the final innervation pattern of the dental papilla and dentin.
These results imply that NGF is required for tooth innervation and that other neurotrophins may also have regulatory roles. Furthermore, the expression patterns of NGF, NT-3, and NT-4/5 as well as neurotrophin receptors suggest that the neurotrophin system may also provide non-neuronal functions during tooth development 18. NGF was also characterized as a potent promoter of mineralization during dentin formation and it also promotes in vitro odontoblast differentiation 26. It has been shown that NT-4 could regulate proliferation and differentiation of the dental epithelium and promote production of the enamel matrix 27.
In study models, human dental pulp cells expressed a neuronal phenotype and produced the neurotrophic factors NGF, GDNF, and BDNF. Dental pulp cells protected primary neurons in in vitro models of Alzheimer’s and Parkinson’s disease and can be considered as possible candidates for studies on cell-based therapy 28. Results of a study showed that neurotrophins speed up dentin sialophosphoprotein (DSPP), ALPase, osteopontin (OPN), type I collagen and bone morphogenetic protein-2 (BMP-2) mRNA expressions and calcified substances formation in cultures of HP cells. In addition, NGF stimulated cell proliferation in cultures of these cells. This shows a new role of neurotrophins in differentiating pulp cells into mineralizing cells in addition to the reported function of the peptides. This study also showed that neurotrophins may be candidates for capping agents, which biologically stimulate reparative dentin formation 29.
Conclusion :
In general, the results of the current study show the expression of neurotrophins in dental pulp tissue, SHED and neural-like cells differentiated from SHED and the highest level of neurotrophins expression in
neural-like cells was observed. Finally, because neurotrophins have neurogenesis, neurotrophic, or neuroprotective effects on the brain, they may have an important role during differentiation of SHED into neural-like cells. Obtained information from this study may lead to a novel method for tooth regeneration.
Acknowledgement :
This study was supported by grants (No; 287112, 287005, 287006) of Dental School, Isfahan University of Medical Sciences.
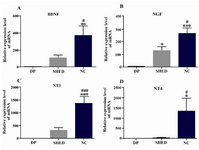
Figure 1. Real time PCR analysis of BDNF, NGF, NT3 and NT4 genes after differentiation of SHED into neural-like cells. All of these neurotrophins increased after differentiation. DP=dental pulp tissue, SHED=Stem cells from human-exfoliated deciduous teeth, NC=neural like cells. Error bars show mean±SD. For each experiment, n=12 for each gene, ***p<0.001, **p<0.01, *p<0.05 in com-parison to dental pulp tissue and ###p<0.001, ##p<0.01, #p<0.05 in comparison to SHED
|

Table 1. Primers and the reaction conditions of RT-PCR
|
|