Mutation Analysis of SLC20A2 and SPP2 as Candidate Genes for Familial Idiopathic Basal Ganglia Calcification
-
Ashtari, Fereshteh
-
Neurology Department, Isfahan Neuroscience Research Centre, Isfahan University of Medical Sciences, Isfahan, Iran
-
Saliminejad, Kioomars
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Ahani, Ali
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Kamali, Koorosh
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Pahlevanzadeh, Zhamak
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
 Khorram Khorshid, Hamid Reza
Genetic Research Center, University of Social Welfare and Rehabilitation Science, Tehran, Iran, Tel: +98 21 22432020; E-mail: hrkk1@uswr.ac.ir
Khorram Khorshid, Hamid Reza
Genetic Research Center, University of Social Welfare and Rehabilitation Science, Tehran, Iran, Tel: +98 21 22432020; E-mail: hrkk1@uswr.ac.ir
-
Genetic Research Center, University of Social Welfare and Rehabilitation Science, Tehran, Iran
Abstract: Background: Familial Idiopathic Basal Ganglia Calcification (IBGC) is a rare neurodegenerative disorder which is usually transmitted as an autosomal dominant trait. IBGC is genetically heterogeneous and SLC20A2, on chromosome 8p21.1–8q11.23, is the first gene found in IBGC-affected patients with varied ancestry. On the other hand, several candidate genes for IBGC on chromosome 2q37, including the SPP2 gene, may play a role in inhibiting calcification.
Methods: Totally, 22 members of a three generational Iranian family affected by IBGC, with an autosomal dominant pattern of inheritance were included in this study. DNA was extracted from the whole blood using standard salting out method. To find a mutation responsible for IBGC, we sequenced the coding region of SLC20A2 as well as promoter and coding region of SPP2 in the index subject of IBGC-affected family.
Results: Pathogenic mutation was found neither in SLC20A2 nor in SPP2.
Conclusion: Our results strengthen genetic heterogeneity of this condition. Additional mutation studies are necessary to find a gene or genes responsible for IBGC in this affected family.
Introduction :
Familial idiopathic basal ganglia calcification (IBGC) is a rare neurodegenerative disease which is usually inherited in an autosomal dominant pattern. Familial IBGC is characterized by bilateral symmetric calcification of brain, especially in basal ganglia, dentate nucleus, thalamus, and centrum semiovale 1. IBGC is a genetically heterogeneous condition and its causative genes and etiology remain unknown at present 2-5.
A whole-genome scan using polymorphic microsatellite markers identified the first chromosomal locus for IBGC (IBGC1) in an American family on chromosome 14q11.2-21.3 6. The IBCG1 locus was excluded in an Italian family from South Tyrol; however, results of a genome-wide scan linkage analysis revealed a novel locus on chromosome 2q37 (IBGC2) in this family 7,8. Several candidate genes for IBGC in the human chromosome 2q37 region including Secreted Phosphoprotein 2 (SPP2) are known to be important for modulating cellular calcium and could thus be interesting for further investigation 8. The secreted phosphoprotein-24 kDa (Spp24), an extracellular matrix protein, which encoded by the SPP2 gene may play a role in inhibiting calcification 8,9.
Another locus showing linkage with IBGC was mapped to the 8p21.1-8q11.23 chromosomal region (IBGC3) in a large Chinese family 3. Further refinement of the IBGC3 locus identified missense mutations, small deletions and splice site mutations in the SLC20A2 gene, encoding the type III sodium-dependent phosphate transporter 2 (PiT2), in IBGC-affected families of varied ancestry 5,10,11.
To find a pathogenic mutation which may be responsible for this condition, we investigated SLC20A2 and SPP2, the two candidate genes for IBGC, in an affected Iranian family.
Materials and Methods :
Subjects: We investigated a three generational Iranian family affected by IBGC, with an autosomal dominant pattern of inheritance (Figure 1). Twenty two family members were included in the present study. Medical histories for the affected members of the pedigree were obtained through existing medical documentation and detailed history. The diagnoses of IBGC were made after evaluation of brain CT scans by a neurologist. Two family members (I-1, II-1) were diagnosed with IBGC according to the medical history. The index subject (II5) was clinically affected and analysis of brain CT scan showed calcification in the basal ganglia and other brain regions. Three symptomatic individuals (II-3, II-9, II-13) were also radiologically affected. Written informed consent was obtained from all participants.
DNA extraction and mutation analysis using direct sequencing: DNA was extracted from whole blood using a standard salting out method 12. Specific primer pairs for the coding region of SLC20A2 as well as promoter and coding region of SPP2 were designed by Primer 3 program. The primers sequences and relative PCR length have been shown in tables 1 and 2. The PCR reactions were carried out in final volume of 25 µl containing: 10×PCR Buffer (Roche, Germany), 1.5 mM MgCl2 (Roche, Germany), 0.4 mM of each dNTP (Fermentas, Germany), 5 pmol of each primer, 50 ng template DNA, 1 U Taq DNA polymerase (Roche, Germany) and sterile distilled water up to 25 µL. Amplification conditions started with an initial denaturation step of 3 min at 94°C, followed by 30 cycles of 30 s denaturation (94°C), 30 s annealing (61°C) and 30 s extension (72°C), ended by a final extension for 7 min (72°C) and final cooling to 4°C. All PCR products were subjected to electrophoresis on 1.5% agarose gel prepared in 1×TAE, stained with ethidium bromide and visualized by exposure to ultraviolet light. To find a pathogenic mutation in the index subject of the pedigree (II-5), the coding region of SLC20A2 and the promoter and coding region of SPP2 were sequenced. Traces were analyzed using Mutation Surveyor software (SoftGenetics). To eliminate false positive results, a mutation was only accepted if it was confirmed by bidirectional sequencing.
PCR-RFLP designing: Sequencing results revealed one polymorphism in the promoter region of the SPP2 gene. For genotyping of the C/T polymorphism (rs13389896) in the promoter region in all family members one PCR-RFLP, with specifically reveres primer, was designed. Briefly, genomic DNA was amplified using forward primer 5’-CCAGGTGATGTGCAAAAGTG-3’ and reverse primer 5’-CTCTATTTTAATTTCATTCTTTGGAAG-3’. The PCR products (290 bp) were digested with restriction enzymes MboII (Fermentase) at 37°C overnight. DNA fragments were subjected to polyacrylamide gel electrophoresis and stained with silver nitrate. C allele was cut into three fragments 135, 66 and 45 bp, while T allele produced four fragments 100, 66, 45 and 35 bp.
Results :
The index subject (II5) is a 54 year old man and analysis of a three generational Iranian family with IBGC showed that ages ranged from 45 to 64 years in the four affected members (II3, II5, II9 and II13). Clinical manifestation of IBGC was not found in the proband’s siblings II7, II11, II15 and II17 with ages of 54, 40, 46 and 41, respectively. The age at onset of clinical symptoms was not available for any of the affected members. The mean age of members in the third generation was 24 years and the oldest person in this generation was a 35 year old man. Clinical manifestation of IBGC has not been observed so far in any of the third generation members.
During the sequencing process of the promoter and coding region of the SPP2 gene in the index subject, no novel mutation was found. We identified a C/T heterozygote mutation at the promoter region of SPP2 (Figure 2). The C/T polymorphism (rs13389896) in the promoter region was analyzed in all family members by PCR-RFLP method. Heterozygous C/T polymorphism was found in both the affected and unaffected members of the family (Figure 1). On the other hand, according to the sequencing results of the index subject, no mutation was found in the coding region of SLC20A2.
Discussion :
IBGC is a genetically heterogeneous disease and the genes in which mutations are responsible for this condition remain unknown 4-6,13. So far, linkage to three loci on chromosome 14q (IBGC1), 2q37 (IBGC2) and 8p21.1-q11.23 (IBGC3) have been identified among American, Italian and Chinese families with IBGC, respectively 3,6,8. However, IBGC1 locus has been excluded in Australian, German, Italian and Serbian IBGC-affected families 2,7,14,15. Several candidate genes for IBGC on chromosome 2q37 region including SPP2 are known to be important for modulating cellular calcium 8.
The human SPP2 gene has been mapped to chromosome 2q37.1 which contains 8 exons and spans about 27 kb of DNA 16. The SPP2 gene codes for an extracellular matrix protein, secreted phosphoprotein-24 kDa (Spp24), which may play a role in inhibiting ectopic calcification 8,9. The Fetuin-Mineral Complex (FMC) is a high molecular mass complex of calcium, phosphate, fetuin, and matrix Gla protein (MGP) and appears to play a critical role in inhibiting calcification in vivo 9. The Spp24 protein is similar in domain structure to fetuin and like fetuin and MGP, contains several residues of phosphoserine. Exogenous Spp24 associated strongly with the FMC when added to serum containing it. These observations suggest that Spp24 may, like fetuin and MGP, play a role in inhibiting calcification 7,9.
During the sequencing process of the promoter and coding region of SPP2 in the index subject of our pedigree, only one C/T heterozygous variation in the promoter region was found. This C/T variation was analyzed in all members of the family, and as a result, heterozygous C/T polymorphism was found in both the affected and unaffected members of the family. According to the dbSNPs 'C' is ancestral allele and 'T' is variant allele with a minor allele frequency (MAF) of 0.23 in the default global population from 1094 worldwide individuals. This polymorphism is common in the general population and seems not to be a pathogenic mutation in IBGC-affected patients. Therefore, SPP2 could be excluded as a candidate gene for IBGC.
The first missense mutation (P521A) associated with IBGC was found in exon 20 of the CTAGE5 gene in a large American family linked to IBGC1 13; however, this mutation was not detected in the two affected Brazilian families as well as in this affected Iranian family 4,17.
Linkage analyses in two Chinese families with IBGC showed a linkage mapping to the 8p21.1-q11.23 chromosomal region (IBGC3) 3,5. Sequencing results of candidate genes within IBGC3 region in the index subjects of these two Chinese families identified a missense mutation in each family in SLC20A2 5. In IBGC-affected families of Chinese, Brazilian and Spanish ancestry, independent mutations including missense mutations and deletion in the four exons of SLC20A2 were identified. This was the first gene linked to IBGC in which mutations are distributed worldwide. It was shown that six out of these seven mutations resulted in considerably impaired inorganic phosphate (Pi) transport of PiT2 in Xenopus laevis oocytes 5. Recently, a novel heterozygous frame shift mutation (c.510delA) within SLC20A2 exon4 was identified in a Chinese family with IBGC 11. In a recent study by Hsu et al, demonstrated mutations in SLC20A2 are a major cause of the disease in 29 IBGC-affected families of varied ancestry 10.
Accordingly, we sequenced the coding region of SLC20A2 in the index subject of our pedigree. None of the previously reported SLC20A2 mutations were found in this person. Wang et al did not find any pathogenic mutation in SLC20A2 in 11 individuals of varied ancestry with either familial or sporadic IBGC. However, their results showed loss-of-function mutations in SLC20A2 for a large subset of IBGC cases 5.
Conclusion :
In conclusion, no pathogenic mutations in the two candidate genes for IBGC, SPP2 and SLC20A2, were found in our pedigree and our result strengthens genetic heterogeneity of this condition. Additional mutation studies and genome wide linkage analysis or exome sequencing are necessary to find gene or genes responsible for IBGC in this affected family.
Acknowledgement :
The authors would like to thank all the family members for their participation in this study.
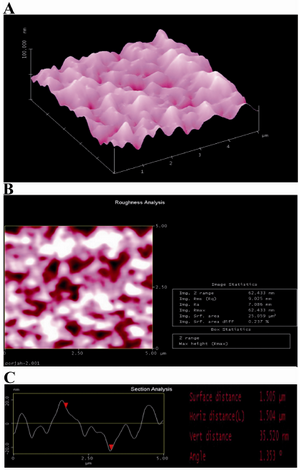
Figure 1. AFM image of agarose-PLL coating: A) three-dimensional image: B) roughness analysis of surface (two-dimensional image) and C) section analysis of agarose-PLL coating
|
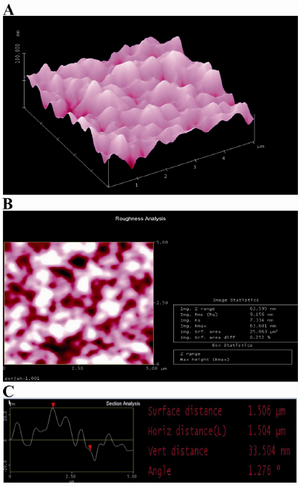
Figure 2. AFM image of agarose layer on unmodified glass: A) three-dimensional image: B) roughness analysis of surface (two-dimensional image) and C) section analysis of agarose layer
|
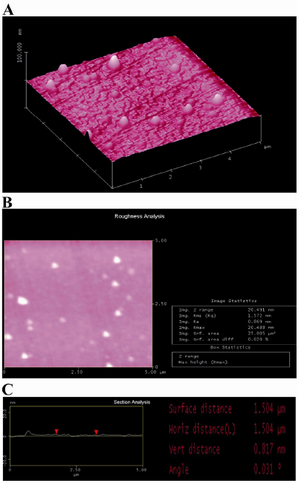
Figure 3. AFM image of PLL coating: A) three-dimensional image; B) roughness analysis of surface (two-dimensional image) and C) section analysis of PLL layer
|
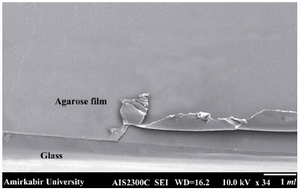
Figure 4. SEM image of agarose layer on the surface of the slides
|
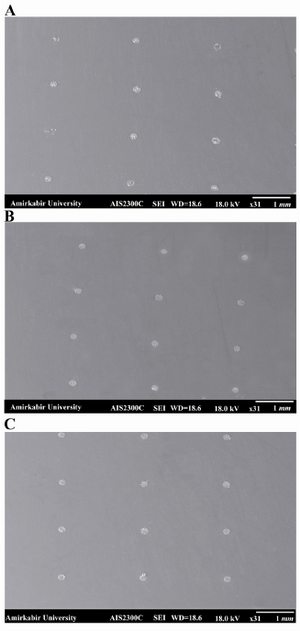
Figure 5. SEM image of the spots written on the A) PLL coated glass slide, the spots were irregular and non-uniform in shape and size; B) agarose coated glass slide, the spots were more regular and uniform in shape and size compared with the spots on PLL coating; C) agarose-PLL coated glass slide, the most uniform and regular spots were formed on agarose- PLL coated slide 4. SEM image of agarose layer on the surface of the slides
|
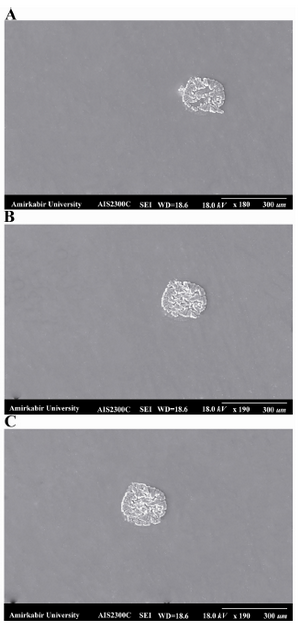
Figure 6. The shape of one spot on the A) PLL coated glass slide, the shape of spot on PLL coated glass was more irregular and had defects in the center of the spot; B) agarose-coated glass slide, the shape of this slide was more desirable than spots on PLL coated glass; C) agarose-PLL coated glass slide. The best round spot was obtained on agarose-PLL coated glass slide
|
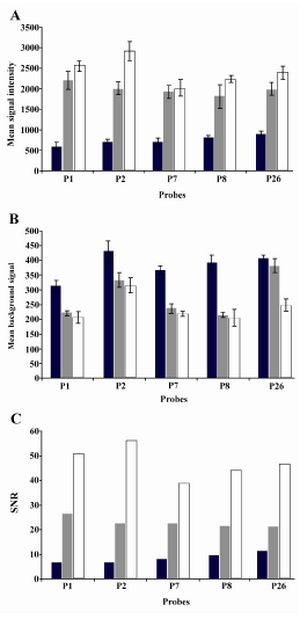
Figure 7. Three different microarray substrates in hybridization reactions. PLL-coated, agarose coated and PLL-agarose coated glass slides were compared in terms of mean signal, mean background signal and signal-to-noise ratio. The highest signal and SNR were obtained for agarose-PLL coated slides. These slides also emitted the lowest background signal for each five probes after hybridization
|
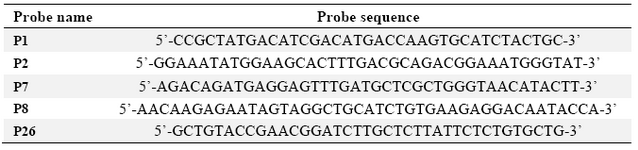
Table 1. Sequences of DNA probes spotted on the fabricated substrates
|
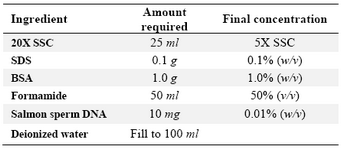
Table 2. Recipe for preparing 100 ml of 1X hybridization solution
|
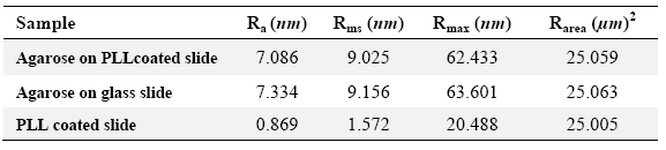
Table 3. Surface analysis data of the samples
|
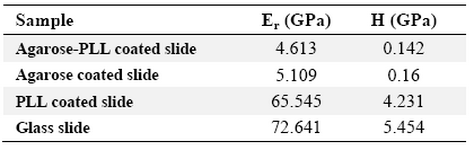
Table 4. Mean reduced elastic modulus and mean hardness of dried samples
|
|