Cloning, Expression and Purification of Penicillin Binding Protein2a (PBP2a) from Methicillin Resistant Staphylococcus aureus : A Study on Immunoreactivity in Balb/C Mouse
-
 Haghighat, Setareh
Department of Biology, Faculty of Basic Sciences, Science and Research branch, Islamic Azad University, Hesarak, Tehran, Iran, Tel: +98 21 47911; E-mail: setareh_haghighat@yahoo.com
Haghighat, Setareh
Department of Biology, Faculty of Basic Sciences, Science and Research branch, Islamic Azad University, Hesarak, Tehran, Iran, Tel: +98 21 47911; E-mail: setareh_haghighat@yahoo.com
-
Department of Biology, Faculty of Basic Sciences, Science and Research branch, Islamic Azad University, Tehran, Iran
-
Rezayat Sorkhabadi, Seyed Mehdi
-
Department of Pharmacology, School of Advanced Sciences and Technology in Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Akhavan Sepahi, Abbas
-
Department of Microbiology, Faculty of Basic Sciences, North Tehran Branch, Islamic Azad University, Tehran, Iran
-
Mahdavi, Mehdi
-
Department of Virology, Pasteur Institute of Iran, Tehran, Iran
Abstract: Background: Staphylococcus aureus (S. aureus) is a major nosocomial pathogen and the infection with this organism in human is increasing due to the spread of antibiotic resistant strains. One of the resistance mechanisms of S. aureus comprises modification in binding proteins to penicillin. Vaccine strategy may be useful in controlling the infections induced by this organism. This study aimed at developing and producing the recombinant protein PBP2a as a vaccine candidate and evaluating the related humoral immune response in a murine model.
Methods: A 242 bp fragment of mecA gene was amplified by PCR from S. aureus COL strain and then cloned into prokaryotic expression vector pET-24a. For expression of recombinant protein, pET24a-mec plasmid was transformed into competent E. coli BL21 (DE3) cells. Recombinant protein was over expressed with 1 mM isopropythio-β-D-galctoside (IPTG) and purified using Ni-NTA agarose. SDS-PAGE and western blotting were carried out to confirm protein expression. For immunization of experimental groups, Balb/c mice were injected subcutaneously with 20 µg of recombinant PBP2a three times with three weeks intervals. The sera of experimental groups were collected three weeks after the last immunization and then specific antibodies were evaluated by ELISA method.
Results: Successful cloning of mecA was confirmed by colony-PCR, enzymatic digestion, and sequencing. SDS-PAGE and western blot analysis showed that recombinant protein with molecular weight of 13 kDa is over expressed. In addition, high titer of specific antibody against PBP2a in vaccinated mice was developed as compared with the control group and confirmed the immunogenicity of the vaccine candidate.
Conclusion: Results suggest that PBP2a recombinant induced specific antibodies and can be used as Staphylococcal vaccine candidate after further studies.
Introduction :
S. aureus is a gram positive bacterium, identified as one of the major nosocomical agents responsible for several hospital-acquired infections, including septic shock, skin infections, and bacteremia 1-3. Hospital-acquired infection is a critical problem especially because Methicillin-resistant Staphylococcus aureus (MRSA) becomes increasingly prevalent 4,5. MRSA is resistant to all ß-lactam antibiotics, due to the presence of an extra penicillin-binding protein (PBP2a) with low affinity to ß-lactam antibiotics 6.
PBP2a is encoded by mecA gene, which is located in a chromosomal cassette of a foreign DNA region integrated into the bacterial chromosome 6-8. PBP2a is classified by Goffin and Ghuysen as a multimodular class B penicillin-binding protein harboring transpeptidase domains 9. While in the presence of ß-lactam antibiotics, normal PBPs are blocked, PBP2a precedes the transpeptidation reactions, thereby results in normal cell wall synthesis 8,10.
Given the inherent and acquired antibiotic resistance of S. aureus, antibiotic therapy for MRSA infections has a limited effectiveness. While vancomycin is the only remaining effective antibiotic 11 against S. aureus, instances of multiresistant bacteria have been frequently reported which won’t be affected by any conventional antibiotics 6,11,12. In addition, the morbidity rate due to MRSA infection can be dependent on status of host immunity, in particular humoral immunity, which is believed to play a significant role against staphylococcal infections 6,13. These ever-increasing obstacles related to conventional antibiotic treatments, point to the need for adopting an alternative approach for effective prevention and treatment of multiresistant bacterial infections 14-16. Vaccine strategy is proven to be useful in controlling such infections 6-8.
Several vaccine strategies against S. aureus have been proposed using bacterial structures 17-19 such as surface polysaccharide 8,13,20 whole cells 8,13 or surface proteins 1 such as clumping factor A 21 and fibronectin-binding protein 22 as target, but none of them revealed protectivity in animal studies and human trials 8. mecA sequence alignments demonstrate a high homology among all MRSA, which substantiates the intention to use this antigen as a vaccine candidate 23. Comparison of the nucleotide and amino acid sequences of mecA among different species has indicated that whereas the N and C-terminal of these sequences are highly conserved, the central hyper variable region is not equally conserved 23. These previous works suggest mecA as an antigen candidate for designing an anti-MRSA vaccine; furthermore, prokaryotic expression system provides a facile method for producing recombinant proteins and may also be useful for the production of PBP2a and other bacterial outer membrane proteins for vaccine studies. The main purpose of the present study was to construct a prokaryotic high level expression system for producing recombinant PBP2a which can be used for vaccine development in future.
Materials and Methods :
Bacterial strains and vector: S. aureus COL strain (methicillin-resistant S. aureus) was kindly obtained from Dr. Mohammad Emaneini (Tehran University of Medical Science). Escherichia coli (E. coli) strains DH5α (Invitrogen, California, USA) and E. coli strains BL21 (DE3) (Novagen, Wisconsin, USA) were used for cloning and expression of recombinant protein, respectively. E. coli cells harboring recombinant plasmids were grown aerobically at 37°C in Luria-Bertani broth (Merck, Darmstadt, Germany) with or without 50 μg/ml kanamycin (Sigma, Saint Louis, MO, USA). Plasmid pET-24a (Novagen, Wisconsin, USA) was used as an expression vector in prokaryotic system.
PCR of mecA gene segment: S. aureus COL strains (methicillin-resistant S. aureus) were cultured on Luria-Bertani (LB) broth and bacteria were collected with centrifugation and then genomic DNA was extracted with mi-Bacterial Genomic DNA isolation kit (Metabion, South Korea) according to the manufacturer’s instructions. A 242 bp (amino acids 370-451) fragment of mecA gene with transpeptidase activity was amplified from genomic DNA as the template. Primer pair used for mecA amplification had the nucleotide sequence as follows: forward primer, containing a restriction site for HindIII (5-’ GGTAAGCTTTTATGTAT GCATGAGTAACGTAAG -3’) and reverse primer with XhoI restriction site (5’- GCCT CGAGACCATTTACCACTTCA TAT CT TG -3’). Pfu DNA polymerase (Fermentase) was used in the reaction. The PCR conditions consisted of 1 cycle of 5 min at 94°C, followed by 35 cycles of 1 min at 94°C, 40 s at 57°C, 1 min at 72°C, and a final cycle of 10 min at 72°C. The PCR products were recovered from the gel and purified by using PCR purification kit (Roche, Germany). The purified mecA fragment was digested with restriction enzymes HindIII and XhoI and ligated into the digested pET-24a vector, which provides six His residues at the C-terminus of the expressed protein. Recombinant vector pET24a-mecA was transformed into the competent E. coli DH5α cells. The integrity of the recovered plasmid was confirmed by colony PCR, restriction endonuclease digestion and sequencing 6-8.
Protein expression: For expression of recombinant protein, pET24a-mecA plasmid was transformed into competent E. coli BL21 (DE3). Transformed cells were grown at 37°C in LB medium containing kanamycin (50 µg/ ml) until exponential phase (OD600 nm=0.6), followed by induction with 1 mM IPTG (Fermentase). Samples were collected every 3 hr for 24 hr and analyzed by SDS-PAGE to follow the best time point of protein expression. SDS-PAGE and western blot analysis were carried out to confirm protein expression 1,6-8,26.
Western blot analysis: For Western blot analysis, the separated proteins by SDS-PAGE gel were transferred to a nitrocellulose membrane (Schleicher & Schuell). The membrane was then blocked in Tris-Buffered Saline (TBS) containing 5% Bovine Serum Albumin (BSA) overnight at 4ºC and washed three times with TBS containing 0.05% Tween 20 (TBST).
Afterwards, the nitrocellulose membrane was incubated for 2 hr at room temperature with mouse anti-histag antibody (Qiagen, USA) diluted 1:10000 in TBS-T. Then the membrane was washed with TBS-T and then incubated with Rabbit anti-mouse immunoglobulin G (heavy and light chain) Horseradish Peroxidase (HRP) conjugate antibody (diluted 1:5000 in TBS-T) for 2 hr at room temperature. After three times of washing, the membrane was treated using DAB solution (Sigma, Saint Louis, MO, USA) and placed in darkness until the appearance of the protein band 26.
Purification of recombinant protein: E. coli BL21 (DE3) containing pET24a-mecA plasmid was grown in large scale and the pellets of bacterial cells expressing protein were harvested and resuspended in lysis buffer (8 M urea, 0.1 M NaH2PO4, and 0.01 M Tris, pH=8.0) containing protease inhibitors. Cell suspension was sonicated and centrifuged for 20 min at 10000 rpm. After centrifugation, the recombinant protein (approximately 13 kDa) was purified from supernatant under denaturing conditions via its His-tag using Ni-nitrilotriacetic acid (Ni-NTA) affinity chromatography (Ni-NTA Agarose; Qiagen) according to the manufacture’s instruction 1,7,23,27. The output fractions were analyzed by SDS-PAGE and the quantity of protein was determined with Bradford protein assay and Nanodrop analyzer. The PBP2a protein solution was dialyzed against 0.1 M phosphate buffered saline (PBS, pH=7.4) for 72 hr to remove urea, filtered (0.22 µm, Sartorius, Germany) and then stored at -70ºC until use 1,7,21,23.
Experimental groups and immunization procedures: Six-to-eight week old female BALB/c mice were purchased from Pasteur Institute of Iran and kept in standard condition. The mice were assigned into two groups (n=14) and immunized subcutaneously with 20 µg of recombinant PBP2a or PBS (the control group) in complete Freund's adjuvant (Sigma, Saint Louis, MO, USA). Booster doses were also given in incomplete Freund’s adjuvant with three and six week intervals. Three weeks after the last immunization, the mice were bled and serum samples were collected and stored at -20°C until use 1,6-8,21,23.
Performing ELISA for specific antibody: The enzyme-linked immunosorbent assay (ELISA) was used to determine the presence of anti-PBP2a antibodies in the sera of immunized mice. Ninety-six well microtiter plates (Extragene, USA) were coated with 100 µl of 10 µ/ml of recombinant protein (1 μg/well) diluted in PBS, and incubated overnight at 4°C. Each plate was washed three times with PBS–T and blocked with PBS containing 5% BSA (blocking buffer) for 2 hr at 37°C. Following blocking and washing, mouse sera were diluted in blocking buffer (1:200 to 1/402240). The plates were incubated for 2 hr at 37°C. Then, the plates were washed three times and incubated with HRP-conjugated anti-mouse IgG (Sigma, USA) diluted 1:7000 (as secondary antibody) at 37°C for 2 hr. To develop the reaction, plates were washed and incubated with TMB (tetramethylbenzidine) as the substrate for 15 min at room temperature in dark condition. The reaction was stopped with 100 µl of 2N H2SO4, and the results were read at optical density of 450 nm (OD450) by ELISA reader 1, 6-8.
Statistical analysis: Data were summarized using descriptive statistical methods. Student’s independent t-test (Statview) and one-way analysis of variance (ANOVA) were used to compare the mean values. A p-value less than 0.05 represented a significant difference. All data were analyzed using SPSS Software Version 20.0 1,6-8.
Results :
Amplification of mecA and construction of pET24a-mecA: Specific primers were designed to amplify mecA from the S. aureus COL strain. The expected size of mecA PCR product, approximately 242 bp, is shown in figure 1. The integrity of the recombinant vector pET24a-mecA was confirmed by double digestion using HindIII and XhoI restriction enzymes (Figure 2) and colony-PCR with specific primers. Identity and orientation of mecA in the construct were confirmed by sequencing the recombinant vector. Cloned mecA gene sequence showed 99.9% homology with reference sequences.
SDS page analysis:Cells harboring pET24a-mecA plasmid were cultured at 37°C in the presence of IPTG. The whole-cell lysates were analyzed by 12% SDS-PAGE. One major band appeared approximately at the 13 kDa position in the case of IPTG induction, which was the expected position of PBP2a (Figure 3). Induction of the cells at 37°C for 6 hr and IPTG with dosage of 1.0 mmol/L was found to be optimal to achieve the highest-level of PBP2a expression. Both supernatant and the pellet of cell lysates were tested for the presence of recombinant proteins. The majority of the expressed protein was detected in inclusion bodies. Therefore, recombinant protein was carefully purified with Ni-NTA affinity chromatography under denaturing condition (Figure 3). This purification yielded 100 mg of highly purified recombinant PBP2a protein from one of induced culture.
Western blot analysis: Western blot analysis was performed to detect the expression of desired protein. The major band observed in SDS-PAGE (13 kDa) (Figure 4) was confirmed as PBP2a protein by western blot analysis with mouse serum anti-his-tag, which indicates the apparent molecular mass of 13 kDa.
Humoral immune response: The specific antibody production was measured by an optimized ELISA method with sera obtained before (pre-immune serum) and after each immunization course with recombinant vaccine. All animals immunized with recombinant vaccine (mecA) were able to produce specific antibodies anti-mecA and the highest antibody titers were observed after the third immunization (second booster). The vaccinated group (Group I) produced higher anti-PBP2a specific antibody after each course of immunization as compared with the control group (after first immunization p=0.023, after second immunization p=0.017, after third immunization p=0.0001) (Figure 5). Result of titration in the experimental group after the third immunization showed that immunization with mecA significantly increased total antibody at the dilution of 1/200 to 1/25600 as compared with the control group (p<0.03) (Figure 6).
Discussion :
Staphylococcal resistance to first-line drugs, including synthetic penicillin and other conventional antibiotics, is a major problem in treating MRSA infection, which is increasingly common, especially in hospitalized patients 7,24. While vancomycin is often used as the only remaining effective antibiotic against MRSA, there are reports that vancomycin resistant species of this infection has emerged 7,11. As there is no effective antibiotic treatment for multiresistant infections, there is a need to develop alternative methods for prevention and treatment of these diseases 6,7,11. In this study, we reported that PBP2 expressed in E. coli BL21 (DE3) can be a potential vaccine candidate.
Being responsible for resistance to all beta-lactam antibiotics, PBP2a was selected as the target protein in developing our potential vaccine candidate 6-10. This enzyme consists of a spanning region which is responsible for its attachment to cytoplasmic membrane, a non-penicillin binding domain of unknown function, and a transpeptidase domain 8,9.
PBP2a is located on the outer surface of the bacterial cell wall, where it can be easily recognized by antibodies raised by host immune system 6-9. In the present study, recombinant DNA technology was used to obtain a high PBP2a production yield. In a similar study by Senna et al 8 a virtually identical fragment of mecA was cloned and expressed using pCI-Neo mammalian vector, in order to achieve a DNA vaccine candidate. Other experiments in which whole mecA gene was used as antigen were reported previously 6,25. While in other studies pET23a and 32a vectors belonging to pET family were used to express mecA gene, 8,25 we used pET24a vector, because of its better expression in comparison with the above mentioned vectors. In addition, pET24 carries six histidine residues in C-terminal that facilitates purification of recombinant protein by Ni NTA agarose 26,27. Since inclusion bodies can be unfolded and require denaturing condition for their solubilization 27, the desired protein was purified in denatured condition effectively and then refolded by dialysis in PBS.
In our study, ELISA results indicated that anti-PBP2a specific antibody was induced. In addition, in this study the titer of antibody was higher than that produced by Roth et al 7, though they used a naked DNA vaccine for immunization.
Humoral immune response is a major factor in the removal of extracellular pathogens such as S. aureus 28. In the presence of this factor, antibody could bind to the surface antigen of the pathogen, activating complement system, and increasing the phagocytosis of the pathogen through opsonization process. PBP2a is able to induce humoral immune response and could eliminate the pathogen with complement activation and induction of opsonization 29.
Conclusion :
In conclusion, our results show that PBP2a can be expressed in E. coli BL21 (DE3) at a high level and stimulate humoral immune response in a murine model. Investigating other aspects of PBP2a as a potential vaccine against MRSA infection, including protectivity effect against bacterial challenge and survival rate is a ground for future studies.
Acknowledgement :
The authors thank Dr. Arash Memarnejad-ian for expert advice and helpful comments relative to this work. This work was supported by Virology and Hepatitis and AIDS Departments of Pasteur Institute of Iran.
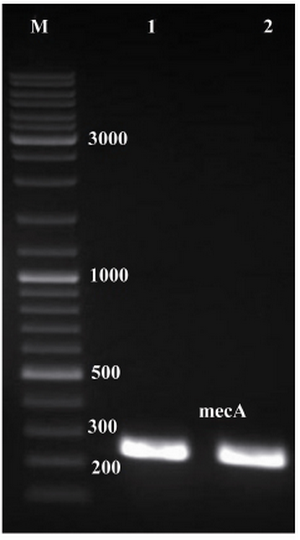
Figure 1. Electrophoresis of PCR products on agarose gel (1% w/v). Lanes 1 and 2, single expected band of mecA (approximately 242 bp); lane M, 1 kB DNA size marker
|
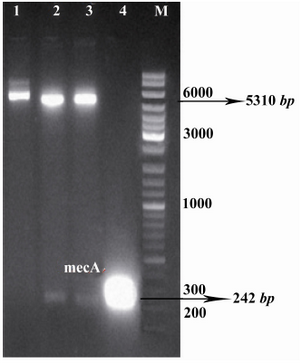
Figure 2. Confirmation of recombinant vector by restriction enzyme digestion. The plasmids were extracted and digested with appropriate restriction enzymes. Lane 1, undigested recombinant vector, pET24a-mecA; lanes 2, 3, recombinant vector, pET24a-mecA, digested with XhoI and HindIII; lane 4, PCR product of mecA gene (approximately 242 bp); lane M, 1 kB DNA size marker. Products were electrophoresed on 1% w/v agarose gel
|
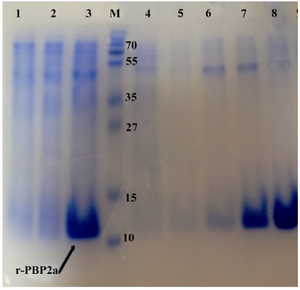
Figure 3. Detection of expressed and purified recombinant PBP2a in SDS-PAGE (12% w/v). The gel was stained with Coomassie blue G-250. Samples were resuspended directly in SDS loading buffer and boiled for 5 min. Amount of proteins loaded in each well was about 50 µg. Lane 1, Negative control cells (BL21 with pET24a(+); lane 2, pellet of un-induced bacteria; lane 3, pellet of IPTG induced bacteria; lane 4, flow through material, lanes 5and 6 inclusion bodies after washing and solubilizing; lane 7, purified r-PBP2a from Ni-NTA Agarose column lane 8, PBP2a protein after dialysis; lane M, standard protein size marker (kDa). The
r-PBP2a proteins have been shown by the arrow
|
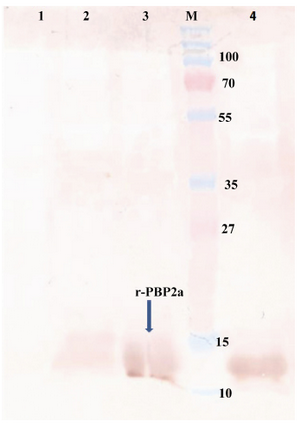
Figure 4. Western blot of recombinant autolysin protein probed by anti-His (1:10,000). Lane 1, control negative (pET24a+ without mecA fragment); lane 2, pellet of un-induced bacteria; lane 3, pellet of IPTG induced bacteria; lane 4, purified r-PBP2a; lane M, pre-stained protein size marker (kDa). HRP-conjugated anti-rabbit IgG (1:7000) and DAB were used
|
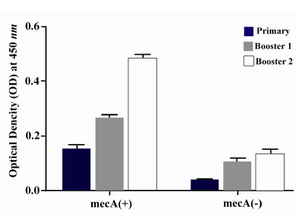
Figure 5. Presence of specific antibodies anti-PBP2a in the sera of mice immunized with the recombinant protein vaccine and the negative controls after each course of immunization. Data presented as mean ±S.D of experimental group (n=14)
|
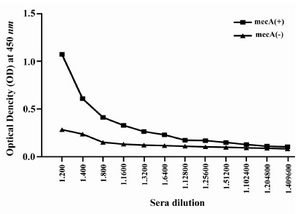
Figure 6. Titration of anti-PBP2a antibody in experimental group with ELISA method. Sera of experimental groups were diluted and ELISA was carried out. Values are presented as mean±S.D of 14 mice in each group
|
|